H34
General
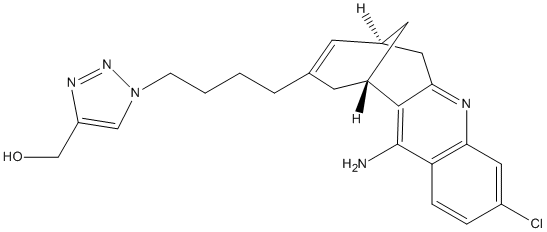
Type : Derivative of Huperzine,Triazol
Chemical_Nomenclature : {1-[4-(12-Amino-3-chloro-6,7,10,11-tetrahydro-7,11-methanocycloocta[b]quinolin-9-yl)butyl]-1H-1,2,3-triazol-4-yl}methanol
Canonical SMILES : C1C2CC3=NC4=C(C=CC(=C4)Cl)C(=C3C1CC(=C2)CCCCN5C=C(N=N5)CO)N
InChI : InChI=1S\/C23H26ClN5O\/c24-17-4-5-19-20(11-17)26-21-10-15-7-14(8-16(9-15)22(21)23(19)25)3-1-2-6-29-12-18(13-30)27-28-29\/h4-5,7,11-12,15-16,30H,1-3,6,8-10,13H2,(H2,25,26)\/t15-,16+\/m0\/s1
InChIKey : MHMXFYDLGYPRNA-JKSUJKDBSA-N
Other name(s) : 4a16
MW : 423.93
Formula : C23H26ClN5O
CAS_number :
PubChem : 56851697
UniChem : MHMXFYDLGYPRNA-JKSUJKDBSA-N
IUPHAR :
Wikipedia :
Target
Families : H34 ligand of proteins in family: ACHE || BCHE
Stucture : 4A16 Structure of mouse Acetylcholinesterase complex with Huprine derivative
Protein : mouse-ACHE
References (1)
| Title : Huprine derivatives as sub-nanomolar human acetylcholinesterase inhibitors: from rational design to validation by X-ray crystallography - Ronco_2012_ChemMedChem_7_400 |
| Author(s) : Ronco C , Carletti E , Colletier JP , Weik M , Nachon F , Jean L , Renard PY |
| Ref : ChemMedChem , 7 :400 , 2012 |
| Abstract : Ronco_2012_ChemMedChem_7_400 |
| ESTHER : Ronco_2012_ChemMedChem_7_400 |
| PubMedSearch : Ronco_2012_ChemMedChem_7_400 |
| PubMedID: 22052791 |
| Gene_locus related to this paper: mouse-ACHE |