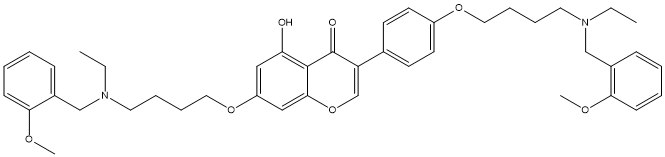
Genistein-O-alkylbenzylamine-25d
25d IC50(M)+/-SD RatAChE 0.09 +/- 0.02, EeAChE 0.14 +/- 0.003, HuAChE 0.35 +/- 0.03, BuChE inhibition(%) 20.0 ORAC, Trolox Equivalents 0.30 +/- 0.01. Genistein, a Phytoestrogen in Soybean Antioxidant, Anti-Inflammatory
General
Type : Multitarget,Monoamine-oxidase-inhibitor,anti-Abeta-aggregation
Chemical_Nomenclature : 7-[4-[ethyl-[(2-methoxyphenyl)methyl]amino]butoxy]-3-[4-[4-[ethyl-[(2-methoxyphenyl)methyl]amino]butoxy]phenyl]-5-hydroxychromen-4-one
Canonical SMILES : CCN(CCCCOC1=CC=C(C=C1)C2=COC3=CC(=CC(=C3C2=O)O)OCCCCN(CC)CC4=CC=CC=C4OC)CC5=CC=CC=C5OC
InChI : InChI=1S\/C43H52N2O7\/c1-5-44(29-33-15-7-9-17-39(33)48-3)23-11-13-25-50-35-21-19-32(20-22-35)37-31-52-41-28-36(27-38(46)42(41)43(37)47)51-26-14-12-24-45(6-2)30-34-16-8-10-18-40(34)49-4\/h7-10,15-22,27-28,31,46H,5-6,11-14,23-26,29-30H2,1-4H3
InChIKey : FFTKMAHTNAEBCM-UHFFFAOYSA-N
Other name(s) : CHEMBL3127003,BDBM50449388
MW : 708.9
Formula : C43H52N2O7
CAS_number :
PubChem : 76329116
UniChem : FFTKMAHTNAEBCM-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : Genistein-O-alkylbenzylamine-25d ligand of proteins in family: ACHE
Stucture :
Protein : human-ACHE
References (2)
| Title : Multifunctional thioxanthone derivatives with acetylcholinesterase, monoamine oxidases and beta-amyloid aggregation inhibitory activities as potential agents against Alzheimer's disease - Luo_2017_Bioorg.Med.Chem_25_1997 |
| Author(s) : Luo L , Li Y , Qiang X , Cao Z , Xu R , Yang X , Xiao G , Song Q , Tan Z , Deng Y |
| Ref : Bioorganic & Medicinal Chemistry , 25 :1997 , 2017 |
| Abstract : Luo_2017_Bioorg.Med.Chem_25_1997 |
| ESTHER : Luo_2017_Bioorg.Med.Chem_25_1997 |
| PubMedSearch : Luo_2017_Bioorg.Med.Chem_25_1997 |
| PubMedID: 28237559 |
| Title : Design, synthesis and evaluation of genistein-O-alkylbenzylamines as potential multifunctional agents for the treatment of Alzheimer's disease - Qiang_2014_Eur.J.Med.Chem_76_314 |
| Author(s) : Qiang X , Sang Z , Yuan W , Li Y , Liu Q , Bai P , Shi Y , Ang W , Tan Z , Deng Y |
| Ref : Eur Journal of Medicinal Chemistry , 76C :314 , 2014 |
| Abstract : Qiang_2014_Eur.J.Med.Chem_76_314 |
| ESTHER : Qiang_2014_Eur.J.Med.Chem_76_314 |
| PubMedSearch : Qiang_2014_Eur.J.Med.Chem_76_314 |
| PubMedID: 24589487 |