GR24
A commonly used synthetic strigolactone analog invented by Gerald Rosebery. Strigolactones (SL) are butenolide plant hormones with tricyclic lactone, that influence root branching leaf shape and senescence. Karrikins (KAR) are different butenolide molecules produced during wildfires in smoke and deposited on soil surface. They are absorbed by seeds and activate germination. KAI2 and D14 are paralogous alpha/beta hydrolase receptors respectively for KARs and SLs they belong to the RsbQ-like family. KAI2 (KARRIKIN-INSENSITVE-2) and D14 (DWARF14) are both receptors and enzymes. The racemic GR24 has both Karrikin and Strigolactone activities as the stereochemistry at the 2' carbon of the butenolide D-ring is identical to the one of 5-deoxystrigol acts as Stigolactone.The other enantiomer acts preferrencially on KAI2 receptor. (+)-GR24 and (-)2'-epi-GR24 have stereochemistry corresponding to taht of natural canonicla SLs of the strigol-type and orobanchol-type respectivly. (-)-GR24 and (+)2'-epi-GR24 stereochemistry is not encountred in natural SLs || IC50 (nM)with YLG as substrate: rac-GR24, AT14 32.9 +/- 6.0; Germinone AT14 12.2 +/- 10; KAI2 585 +/- 36; dMgerminone AT14 162 +/- 9.0; KAI2 46.4 +/- 3.0
General
Type : Strigolactone,Strigolactone receptors ligand,Inden
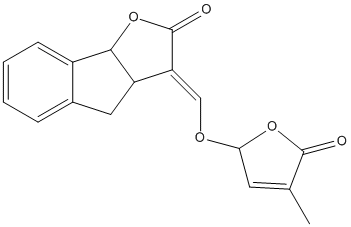
Chemical_Nomenclature : (3E)-3-[(4-methyl-5-oxo-2H-furan-2-yl)oxymethylidene]-4,8b-dihydro-3aH-indeno[1,2-b]furan-2-one
Canonical SMILES : CC1=CC(OC1=O)OC=C2C3CC4=CC=CC=C4C3OC2=O
InChI : InChI=1S\/C17H14O5\/c1-9-6-14(21-16(9)18)20-8-13-12-7-10-4-2-3-5-11(10)15(12)22-17(13)19\/h2-6,8,12,14-15H,7H2,1H3\/b13-8+
InChIKey : XHSDUVBUZOUAOQ-MDWZMJQESA-N || XHSDUVBUZOUAOQ-WSTIHDONSA-N || XHSDUVBUZOUAOQ-IMBONUEFSA-N
Other name(s) : SCHEMBL9983619,CHEMBL2447920,AC1MI1TT,(+)-epi-GR-24,SCHEMBL13974737,ZINC33753817,PL021299,dGR24ent-5DS
MW : 298.29
Formula : C17H14O5
CAS_number :
PubChem : 11748521, 3036799, 102518383
UniChem : XHSDUVBUZOUAOQ-MDWZMJQESA-N || XHSDUVBUZOUAOQ-WSTIHDONSA-N || XHSDUVBUZOUAOQ-IMBONUEFSA-N
Wikipedia : Strigolactone
Target
Families : GR24 ligand of proteins in family
RsbQ-like
Fungal-D14-Strigolactone-R
Protein :
arath-AT5G62180
crypa-CpD14
bacsu-RsbQ
References (10)
| Title : Perception of butenolides by Bacillus subtilis via the alpha\/beta hydrolase RsbQ - Melville_2023_Curr.Biol__ |
| Author(s) : Melville KT , Kamran M , Yao J , Costa M , Holland M , Taylor NL , Fritz G , Flematti GR , Waters MT |
| Ref : Current Biology , : , 2023 |
| Abstract : Melville_2023_Curr.Biol__ |
| ESTHER : Melville_2023_Curr.Biol__ |
| PubMedSearch : Melville_2023_Curr.Biol__ |
| PubMedID: 38183985 |
| Gene_locus related to this paper: bacsu-RsbQ |
| Title : A structural homologue of the plant receptor D14 mediates responses to strigolactones in the fungal phytopathogen Cryphonectria parasitica - Fiorilli_2022_New.Phytol__ |
| Author(s) : Fiorilli V , Forgia M , de Saint Germain A , D'Arrigo G , Cornu D , Le Bris P , Al-Babili S , Cardinale F , Prandi C , Spyrakis F , Boyer FD , Turina M , Lanfranco L |
| Ref : New Phytol , : , 2022 |
| Abstract : Fiorilli_2022_New.Phytol__ |
| ESTHER : Fiorilli_2022_New.Phytol__ |
| PubMedSearch : Fiorilli_2022_New.Phytol__ |
| PubMedID: 35119708 |
| Gene_locus related to this paper: crypa-CpD14 |
| Title : Desmethyl butenolides are optimal ligands for karrikin receptor proteins - Yao_2021_New.Phytol__ |
| Author(s) : Yao J , Scaffidi A , Meng Y , Melville KT , Komatsu A , Khosla A , Nelson DC , Kyozuka J , Flematti GR , Waters MT |
| Ref : New Phytol , : , 2021 |
| Abstract : Yao_2021_New.Phytol__ |
| ESTHER : Yao_2021_New.Phytol__ |
| PubMedSearch : Yao_2021_New.Phytol__ |
| PubMedID: 33474738 |
| Gene_locus related to this paper: arath-AtD14 , arath-KAI2.D14L |
| Title : Strigolactone promotes cytokinin degradation through transcriptional activation of CYTOKININ OXIDASE\/DEHYDROGENASE 9 in rice - Duan_2019_Proc.Natl.Acad.Sci.U.S.A_116_14319 |
| Author(s) : Duan J , Yu H , Yuan K , Liao Z , Meng X , Jing Y , Liu G , Chu J , Li J |
| Ref : Proc Natl Acad Sci U S A , 116 :14319 , 2019 |
| Abstract : Duan_2019_Proc.Natl.Acad.Sci.U.S.A_116_14319 |
| ESTHER : Duan_2019_Proc.Natl.Acad.Sci.U.S.A_116_14319 |
| PubMedSearch : Duan_2019_Proc.Natl.Acad.Sci.U.S.A_116_14319 |
| PubMedID: 31235564 |
| Title : An allelic series at the KARRIKIN INSENSITIVE 2 locus of Arabidopsis thaliana decouples ligand hydrolysis and receptor degradation from downstream signalling - Yao_2018_Plant.J_96_75 |
| Author(s) : Yao J , Mashiguchi K , Scaffidi A , Akatsu T , Melville KT , Morita R , Morimoto Y , Smith SM , Seto Y , Flematti GR , Yamaguchi S , Waters MT |
| Ref : Plant J , 96 :75 , 2018 |
| Abstract : Yao_2018_Plant.J_96_75 |
| ESTHER : Yao_2018_Plant.J_96_75 |
| PubMedSearch : Yao_2018_Plant.J_96_75 |
| PubMedID: 29982999 |
| Gene_locus related to this paper: arath-KAI2.D14L |
| Title : Smoke and Hormone Mirrors: Action and Evolution of Karrikin and Strigolactone Signaling - Morffy_2016_Trends.Genet_32_176 |
| Author(s) : Morffy N , Faure L , Nelson DC |
| Ref : Trends Genet , 32 :176 , 2016 |
| Abstract : Morffy_2016_Trends.Genet_32_176 |
| ESTHER : Morffy_2016_Trends.Genet_32_176 |
| PubMedSearch : Morffy_2016_Trends.Genet_32_176 |
| PubMedID: 26851153 |
| Title : Destabilization of strigolactone receptor DWARF14 by binding of ligand and E3-ligase signaling effector DWARF3 - Zhao_2015_Cell.Res_25_1219 |
| Author(s) : Zhao LH , Zhou XE , Yi W , Wu Z , Liu Y , Kang Y , Hou L , de Waal PW , Li S , Jiang Y , Scaffidi A , Flematti GR , Smith SM , Lam VQ , Griffin PR , Wang Y , Li J , Melcher K , Xu HE |
| Ref : Cell Res , 25 :1219 , 2015 |
| Abstract : Zhao_2015_Cell.Res_25_1219 |
| ESTHER : Zhao_2015_Cell.Res_25_1219 |
| PubMedSearch : Zhao_2015_Cell.Res_25_1219 |
| PubMedID: 26470846 |
| Gene_locus related to this paper: orysj-Q10QA5 |
| Title : Detection of Parasitic Plant Suicide Germination Compounds Using a High-Throughput Arabidopsis HTL\/KAI2 Strigolactone Perception System - Toh_2014_Chem.Biol_21_988 |
| Author(s) : Toh S , Holbrook-Smith D , Stokes ME , Tsuchiya Y , McCourt P |
| Ref : Chemical Biology , 21 :988 , 2014 |
| Abstract : Toh_2014_Chem.Biol_21_988 |
| ESTHER : Toh_2014_Chem.Biol_21_988 |
| PubMedSearch : Toh_2014_Chem.Biol_21_988 |
| PubMedID: 25126711 |
| Title : Strigolactone Hormones and Their Stereoisomers Signal through Two Related Receptor Proteins to Induce Different Physiological Responses in Arabidopsis - Scaffidi_2014_Plant.Physiol_165_1221 |
| Author(s) : Scaffidi A , Waters MT , Sun YK , Skelton BW , Dixon KW , Ghisalberti EL , Flematti GR , Smith SM |
| Ref : Plant Physiol , 165 :1221 , 2014 |
| Abstract : Scaffidi_2014_Plant.Physiol_165_1221 |
| ESTHER : Scaffidi_2014_Plant.Physiol_165_1221 |
| PubMedSearch : Scaffidi_2014_Plant.Physiol_165_1221 |
| PubMedID: 24808100 |
| Title : The preparation of synthetic analogues of strigol - Johnson_1981_J.Chem.Soc.Perkin.1__1734 |
| Author(s) : Johnson AW , Gowda G , Wassanali A , Knox J , Monaco S , Razawi Z , Roseberry G |
| Ref : J Chem Soc Perkin 1 :1734 , 1981 |
| Abstract : Johnson_1981_J.Chem.Soc.Perkin.1__1734 |
| ESTHER : Johnson_1981_J.Chem.Soc.Perkin.1__1734 |
| PubMedSearch : Johnson_1981_J.Chem.Soc.Perkin.1__1734 |
| PubMedID: |