FPB-derived-7k
Reversed Amide Inhibitor: reverse binding to DPP-4 when compared to classical DPP-4 inhibitors
General
Type : Pyrrolidine,Sulfur Compound
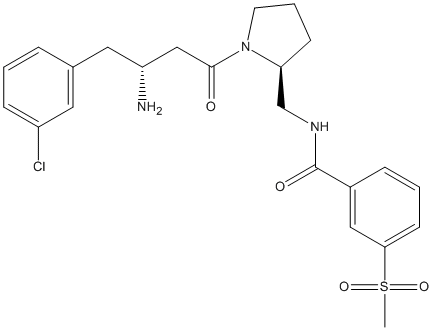
Chemical_Nomenclature : N-[[(2S)-1-[(3R)-3-amino-4-(3-chlorophenyl)butanoyl]pyrrolidin-2-yl]methyl]-3-methylsulfonylbenzamide
Canonical SMILES : CS(=O)(=O)C1=CC=CC(=C1)C(=O)NCC2CCCN2C(=O)CC(CC3=CC(=CC=C3)Cl)N
InChI : InChI=1S\/C23H28ClN3O4S\/c1-32(30,31)21-9-3-6-17(13-21)23(29)26-15-20-8-4-10-27(20)22(28)14-19(25)12-16-5-2-7-18(24)11-16\/h2-3,5-7,9,11,13,19-20H,4,8,10,12,14-15,25H2,1H3,(H,26,29)\/t19-,20+\/m1\/s1
InChIKey : QRGBOABBMKYMLG-UXHICEINSA-N
Other name(s) : PS4,CHEMBL564249,N-({(2s)-1-[(3r)-3-Amino-4-(3-Chlorophenyl)butanoyl]pyrrolidin-2-Yl}methyl)-3-(Methylsulfonyl)benzamide,SCHEMBL8289096,BDBM50296131,DB08429,N-(((S)-1-((R)-3-amino-4-(3-chlorophenyl)butanoyl)pyrrolidin-2-yl)methyl)-3-(methylsulfonyl)benzamide
MW : 478.00
Formula : C23H28ClN3O4S
CAS_number :
PubChem : 42608447
UniChem : QRGBOABBMKYMLG-UXHICEINSA-N
IUPHAR :
Wikipedia :
Target
Families : FPB-derived-7k ligand of proteins in family: DPP4N_Peptidase_S9
Stucture : 3H0C Crystal Structure of Human Dipeptidyl Peptidase IV (CD26) in Complex with a Reversed Amide Inhibitor
Protein : human-DPP4
References (1)
| Title : Discovery of beta-homophenylalanine based pyrrolidin-2-ylmethyl amides and sulfonamides as highly potent and selective inhibitors of dipeptidyl peptidase IV - Nordhoff_2009_Bioorg.Med.Chem.Lett_19_4201 |
| Author(s) : Nordhoff S , Cerezo-Galvez S , Deppe H , Hill O , Lopez-Canet M , Rummey C , Thiemann M , Matassa VG , Edwards PJ , Feurer A |
| Ref : Bioorganic & Medicinal Chemistry Lett , 19 :4201 , 2009 |
| Abstract : Nordhoff_2009_Bioorg.Med.Chem.Lett_19_4201 |
| ESTHER : Nordhoff_2009_Bioorg.Med.Chem.Lett_19_4201 |
| PubMedSearch : Nordhoff_2009_Bioorg.Med.Chem.Lett_19_4201 |
| PubMedID: 19515557 |
| Gene_locus related to this paper: human-DPP4 |