FEPAD
IC50 values for hMAGL-expressing cell lines 77.6 +/- 9.2 nM and recombinant hMAGL 23.8 +/- 4.9 nM. A superior to 100-fold selectivity over other major serine hydrolases, including FAAH, ABHD6, ABHD12, and KIAA1363, and CB1/CB2 receptors. F-18 labeled radioligand [18F]FEPAD is a PET probe for non-invasive assessment of MAGL brown adipose tissue
General
Type : Piperazine,Azetidin,Trifluoro,Thiazolidine,Sulfur Compound,PET probe
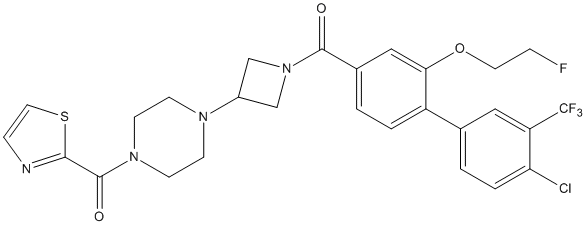
Chemical_Nomenclature : (4-(1-(4'-chloro-2-(2-fluoroethoxy)-3'-(trifluoromethyl)-[1,1'-biphenyl]-4- carbonyl)azetidin-3-yl)piperazin -1-yl)(thiazol-2-yl)methanone
Canonical SMILES : C1CN(CCN1C2CN(C2)C(=O)C3=CC=C(C(=C3)OCCF)C4=CC=C(C(=C4)C(F)(F)F)Cl)C(C5=NC=CS5)=O
InChI : InChI=1S\/C27H25ClF4N4O3S\/c28-22-4-2-17(13-21(22)27(30,31)32)20-3-1-18(14-23(20)39-11-5-29)25(37)36-15-19(16-36)34-7-9-35(10-8-34)26(38)24-33-6-12-40-24\/h1-4,6,12-14,19H,5,7-11,15-16H2
InChIKey : CUWKHRITMRZSAU-UHFFFAOYSA-N
Other name(s) :
MW : 597.O2
Formula : C27H25ClF4N4O3S
CAS_number :
PubChem :
UniChem : CUWKHRITMRZSAU-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : FEPAD ligand of proteins in family: Monoglyceridelipase_lysophospholip
Stucture :
Protein : human-MGLL
References (1)
| Title : A novel monoacylglycerol lipase-targeted (18)F-labeled probe for positron emission tomography imaging of brown adipose tissue in the energy network - Cheng_2022_Acta.Pharmacol.Sin__ |
| Author(s) : Cheng R , Fujinaga M , Yang J , Rong J , Haider A , Ogasawara D , Van RS , Shao T , Chen Z , Zhang X , Calderon Leon ER , Zhang Y , Mori W , Kumata K , Yamasaki T , Xie L , Sun S , Wang L , Ran C , Shao Y , Cravatt B , Josephson L , Zhang MR , Liang SH |
| Ref : Acta Pharmacol Sin , : , 2022 |
| Abstract : Cheng_2022_Acta.Pharmacol.Sin__ |
| ESTHER : Cheng_2022_Acta.Pharmacol.Sin__ |
| PubMedSearch : Cheng_2022_Acta.Pharmacol.Sin__ |
| PubMedID: 35513432 |
| Gene_locus related to this paper: human-MGLL |