Evogliptin
Derivative of DA-1266. DDP4 IC50 0.666 nM
General
Type : Drug,Gliptin,Trifluoro
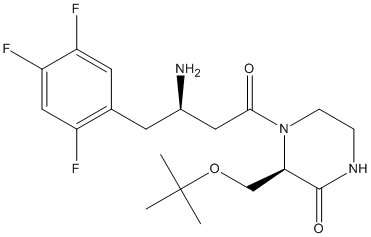
Chemical_Nomenclature : (3R)-4-[(3R)-3-amino-4-(2,4,5-trifluorophenyl)butanoyl]-3-[(2-methylpropan-2-yl)oxymethyl]piperazin-2-one
Canonical SMILES : CC(C)(C)OCC1C(=O)NCCN1C(=O)CC(CC2=CC(=C(C=C2F)F)F)N
InChI : InChI=1S\/C19H26F3N3O3\/c1-19(2,3)28-10-16-18(27)24-4-5-25(16)17(26)8-12(23)6-11-7-14(21)15(22)9-13(11)20\/h7,9,12,16H,4-6,8,10,23H2,1-3H3,(H,24,27)\/t12-,16-\/m1\/s1
InChIKey : LCDDAGSJHKEABN-MLGOLLRUSA-N
Other name(s) : 8VU,DA-1229,DA1229,UNII-09118300L7,CHEMBL1779710
MW : 401.43
Formula : C19H26F3N3O3
CAS_number : 1222102-29-5
PubChem : 25022354
UniChem : LCDDAGSJHKEABN-MLGOLLRUSA-N
IUPHAR :
Wikipedia : Evogliptin
Target
Families : Evogliptin ligand of proteins in family: DPP4N_Peptidase_S9
Stucture : 5Y7K Crystal structure of human DPP4 in complex with inhibitor Evogliptin
Protein : human-DPP4
References (20)
| Title : Cardiovascular safety of evogliptin dual and triple therapy in patients with type 2 diabetes: a nationwide cohort study - Park_2024_BMJ.Open_14_e077084 |
| Author(s) : Park S , Jeong HE , Oh IS , Hong S , Yu SH , Lee CB , Shin JY |
| Ref : BMJ Open , 14 :e077084 , 2024 |
| Abstract : Park_2024_BMJ.Open_14_e077084 |
| ESTHER : Park_2024_BMJ.Open_14_e077084 |
| PubMedSearch : Park_2024_BMJ.Open_14_e077084 |
| PubMedID: 38626972 |
| Title : Evogliptin, a DPP-4 inhibitor, prevents diabetic cardiomyopathy by alleviating cardiac lipotoxicity in db\/db mice - Pham_2023_Exp.Mol.Med__ |
| Author(s) : Pham TK , Nguyen THT , Yi JM , Kim GS , Yun HR , Kim HK , Won JC |
| Ref : Exp Mol Med , : , 2023 |
| Abstract : Pham_2023_Exp.Mol.Med__ |
| ESTHER : Pham_2023_Exp.Mol.Med__ |
| PubMedSearch : Pham_2023_Exp.Mol.Med__ |
| PubMedID: 37009790 |
| Title : Cardiovascular safety of evogliptin in patients with type 2 diabetes mellitus: a nationwide cohort study - Park_2021_Diabetes.Obes.Metab__ |
| Author(s) : Park SH , Jeong HE , Oh IS , Hong SM , Yu SH , Lee CB , Shin JY |
| Ref : Diabetes Obes Metab , : , 2021 |
| Abstract : Park_2021_Diabetes.Obes.Metab__ |
| ESTHER : Park_2021_Diabetes.Obes.Metab__ |
| PubMedSearch : Park_2021_Diabetes.Obes.Metab__ |
| PubMedID: 33502058 |
| Title : Clinical Evidence of Evogliptin plus Metformin in Management of Type 2 Diabetes mellitus - Bajaj_2021_J.Assoc.Physicians.India_69_25 |
| Author(s) : Bajaj S , Aiwale A , Trailokya A , Sharma A |
| Ref : J Assoc Physicians India , 69 :25 , 2021 |
| Abstract : Bajaj_2021_J.Assoc.Physicians.India_69_25 |
| ESTHER : Bajaj_2021_J.Assoc.Physicians.India_69_25 |
| PubMedSearch : Bajaj_2021_J.Assoc.Physicians.India_69_25 |
| PubMedID: 33527807 |
| Title : Pharmacokinetics and safety of evogliptin in hepatically impaired patients - Hong_2021_Br.J.Clin.Pharmacol_87_2757 |
| Author(s) : Hong T , Jin BH , Kim CO , Yoo BW , Kim D , Lee JI , Kim BK , Ahn SH , Kim DY , Park JY , Park MS |
| Ref : British Journal of Clinical Pharmacology , 87 :2757 , 2021 |
| Abstract : Hong_2021_Br.J.Clin.Pharmacol_87_2757 |
| ESTHER : Hong_2021_Br.J.Clin.Pharmacol_87_2757 |
| PubMedSearch : Hong_2021_Br.J.Clin.Pharmacol_87_2757 |
| PubMedID: 33245796 |
| Title : Unique binding mode of Evogliptin with human dipeptidyl peptidase IV - Lee_2017_Biochem.Biophys.Res.Commun_494_452 |
| Author(s) : Lee HK , Kim MK , Kim HD , Kim HJ , Kim JW , Lee JO , Kim CW , Kim EE |
| Ref : Biochemical & Biophysical Research Communications , 494 :452 , 2017 |
| Abstract : Lee_2017_Biochem.Biophys.Res.Commun_494_452 |
| ESTHER : Lee_2017_Biochem.Biophys.Res.Commun_494_452 |
| PubMedSearch : Lee_2017_Biochem.Biophys.Res.Commun_494_452 |
| PubMedID: 29061303 |
| Gene_locus related to this paper: human-DPP4 |
| Title : In vitro evaluation of potential transporter-mediated drug interactions of evogliptin - Lee_2017_Biopharm.Drug.Dispos_38_398 |
| Author(s) : Lee DY , Chae HW , Shim HJ |
| Ref : Biopharmaceutics & Drug Disposition , 38 :398 , 2017 |
| Abstract : Lee_2017_Biopharm.Drug.Dispos_38_398 |
| ESTHER : Lee_2017_Biopharm.Drug.Dispos_38_398 |
| PubMedSearch : Lee_2017_Biopharm.Drug.Dispos_38_398 |
| PubMedID: 28503751 |
| Title : Effects of renal impairment on the pharmacokinetics and pharmacodynamics of a novel dipeptidyl peptidase-4 inhibitor, evogliptin (DA-1229) - Oh_2017_Diabetes.Obes.Metab_19_294 |
| Author(s) : Oh J , Kim AH , Lee S , Cho H , Kim YS , Bahng MY , Yoon SH , Cho JY , Jang IJ , Yu KS |
| Ref : Diabetes Obes Metab , 19 :294 , 2017 |
| Abstract : Oh_2017_Diabetes.Obes.Metab_19_294 |
| ESTHER : Oh_2017_Diabetes.Obes.Metab_19_294 |
| PubMedSearch : Oh_2017_Diabetes.Obes.Metab_19_294 |
| PubMedID: 27761990 |
| Title : Effects of clarithromycin on the pharmacokinetics of evogliptin in healthy volunteers - Oh_2017_J.Clin.Pharm.Ther_42_689 |
| Author(s) : Oh ES , Choi C , Kim CO , Kim KH , Kim YN , Kim SJ , Park MS |
| Ref : J Clin Pharm Ther , 42 :689 , 2017 |
| Abstract : Oh_2017_J.Clin.Pharm.Ther_42_689 |
| ESTHER : Oh_2017_J.Clin.Pharm.Ther_42_689 |
| PubMedSearch : Oh_2017_J.Clin.Pharm.Ther_42_689 |
| PubMedID: 28806472 |
| Title : Efficacy and safety of evogliptin monotherapy in patients with type 2 diabetes and moderately elevated glycated haemoglobin levels after diet and exercise - Park_2017_Diabetes.Obes.Metab_19_1681 |
| Author(s) : Park J , Park SW , Yoon KH , Kim SR , Ahn KJ , Lee JH , Mok JO , Chung CH , Han KA , Koh GP , Kang JG , Lee CB , Kim SH , Kwon NY , Kim DM |
| Ref : Diabetes Obes Metab , 19 :1681 , 2017 |
| Abstract : Park_2017_Diabetes.Obes.Metab_19_1681 |
| ESTHER : Park_2017_Diabetes.Obes.Metab_19_1681 |
| PubMedSearch : Park_2017_Diabetes.Obes.Metab_19_1681 |
| PubMedID: 28448688 |
| Title : Efficacy and safety of adding evogliptin versus sitagliptin for metformin-treated patients with type 2 diabetes: A 24-week randomized, controlled trial with open label extension - Hong_2017_Diabetes.Obes.Metab_19_654 |
| Author(s) : Hong SM , Park CY , Hwang DM , Han KA , Lee CB , Chung CH , Yoon KH , Mok JO , Park KS , Park SW |
| Ref : Diabetes Obes Metab , 19 :654 , 2017 |
| Abstract : Hong_2017_Diabetes.Obes.Metab_19_654 |
| ESTHER : Hong_2017_Diabetes.Obes.Metab_19_654 |
| PubMedSearch : Hong_2017_Diabetes.Obes.Metab_19_654 |
| PubMedID: 28058750 |
| Title : Prevention and treatment effect of evogliptin on hepatic steatosis in high-fat-fed animal models - Kim_2017_Arch.Pharm.Res_40_268 |
| Author(s) : Kim MK , Chae YN , Ahn GJ , Shin CY , Choi SH , Yang EK , Sohn YS , Son MH |
| Ref : Arch Pharm Res , 40 :268 , 2017 |
| Abstract : Kim_2017_Arch.Pharm.Res_40_268 |
| ESTHER : Kim_2017_Arch.Pharm.Res_40_268 |
| PubMedSearch : Kim_2017_Arch.Pharm.Res_40_268 |
| PubMedID: 27885461 |
| Title : Pharmacokinetic comparison using two tablets of an evogliptin\/metformin XR 2.5\/500 mg fixed dose combination vs. 1 tablet each of evogliptin 5 mg and metformin XR 1,000 mg - Yoon_2017_Int.J.Clin.Pharmacol.Ther_55_533 |
| Author(s) : Yoon S , Rhee SJ , Park SI , Yoon SH , Cho JY , Jang IJ , Lee S , Yu KS |
| Ref : Int J Clinical Pharmacology & Therapeutics , 55 :533 , 2017 |
| Abstract : Yoon_2017_Int.J.Clin.Pharmacol.Ther_55_533 |
| ESTHER : Yoon_2017_Int.J.Clin.Pharmacol.Ther_55_533 |
| PubMedSearch : Yoon_2017_Int.J.Clin.Pharmacol.Ther_55_533 |
| PubMedID: 28406090 |
| Title : Evogliptin: a new dipeptidyl peptidase inhibitor for the treatment of type 2 diabetes - Tan_2016_Expert.Opin.Pharmacother_17_1285 |
| Author(s) : Tan X , Hu J |
| Ref : Expert Opin Pharmacother , 17 :1285 , 2016 |
| Abstract : Tan_2016_Expert.Opin.Pharmacother_17_1285 |
| ESTHER : Tan_2016_Expert.Opin.Pharmacother_17_1285 |
| PubMedSearch : Tan_2016_Expert.Opin.Pharmacother_17_1285 |
| PubMedID: 27156529 |
| Title : Pharmacokinetics of the evogliptin\/metformin extended-release (5\/1,000 mg) fixed-dose combination formulation compared to the corresponding loose combination, and food effect in healthy subjects - Rhee_2016_Drug.Des.Devel.Ther_10_1411 |
| Author(s) : Rhee SJ , Lee S , Yoon SH , Cho JY , Jang IJ , Yu KS |
| Ref : Drug Des Devel Ther , 10 :1411 , 2016 |
| Abstract : Rhee_2016_Drug.Des.Devel.Ther_10_1411 |
| ESTHER : Rhee_2016_Drug.Des.Devel.Ther_10_1411 |
| PubMedSearch : Rhee_2016_Drug.Des.Devel.Ther_10_1411 |
| PubMedID: 27110098 |
| Title : Pharmacokinetic and pharmacodynamic interactions between metformin and a novel dipeptidyl peptidase-4 inhibitor, evogliptin, in healthy subjects - Rhee_2016_Drug.Des.Devel.Ther_10_2525 |
| Author(s) : Rhee SJ , Choi Y , Lee S , Oh J , Kim SJ , Yoon SH , Cho JY , Yu KS |
| Ref : Drug Des Devel Ther , 10 :2525 , 2016 |
| Abstract : Rhee_2016_Drug.Des.Devel.Ther_10_2525 |
| ESTHER : Rhee_2016_Drug.Des.Devel.Ther_10_2525 |
| PubMedSearch : Rhee_2016_Drug.Des.Devel.Ther_10_2525 |
| PubMedID: 27570447 |
| Title : Hepatic role in an early glucose-lowering effect by a novel dipeptidyl peptidase 4 inhibitor, evogliptin, in a rodent model of type 2 diabetes - Kim_2016_Eur.J.Pharmacol_771_65 |
| Author(s) : Kim TH , Kim MK , Cheong YH , Chae YN , Lee Y , Ka SO , Jung IH , Shin CY , Bae EJ , Son MH |
| Ref : European Journal of Pharmacology , 771 :65 , 2016 |
| Abstract : Kim_2016_Eur.J.Pharmacol_771_65 |
| ESTHER : Kim_2016_Eur.J.Pharmacol_771_65 |
| PubMedSearch : Kim_2016_Eur.J.Pharmacol_771_65 |
| PubMedID: 26621343 |
| Title : In Vitro Metabolic Pathways of the New Anti-Diabetic Drug Evogliptin in Human Liver Preparations - Jeong_2015_Molecules_20_21802 |
| Author(s) : Jeong HU , Kim JH , Lee DY , Shim HJ , Lee HS |
| Ref : Molecules , 20 :21802 , 2015 |
| Abstract : Jeong_2015_Molecules_20_21802 |
| ESTHER : Jeong_2015_Molecules_20_21802 |
| PubMedSearch : Jeong_2015_Molecules_20_21802 |
| PubMedID: 26690104 |
| Title : Evogliptin: First Global Approval - McCormack_2015_Drugs_75_2045 |
| Author(s) : McCormack PL |
| Ref : Drugs , 75 :2045 , 2015 |
| Abstract : McCormack_2015_Drugs_75_2045 |
| ESTHER : McCormack_2015_Drugs_75_2045 |
| PubMedSearch : McCormack_2015_Drugs_75_2045 |
| PubMedID: 26541763 |
| Title : Multiple-dose pharmacokinetics and pharmacodynamics of evogliptin (DA-1229), a novel dipeptidyl peptidase IV inhibitor, in healthy volunteers - Gu_2014_Drug.Des.Devel.Ther_8_1709 |
| Author(s) : Gu N , Park MK , Kim TE , Bahng MY , Lim KS , Cho SH , Yoon SH , Cho JY , Jang IJ , Yu KS |
| Ref : Drug Des Devel Ther , 8 :1709 , 2014 |
| Abstract : Gu_2014_Drug.Des.Devel.Ther_8_1709 |
| ESTHER : Gu_2014_Drug.Des.Devel.Ther_8_1709 |
| PubMedSearch : Gu_2014_Drug.Des.Devel.Ther_8_1709 |
| PubMedID: 25336915 |