Evodiamine
General
Type : Derivative of Evodiamine,Natural,beta-carboline,Not A\/B H target,Multitarget
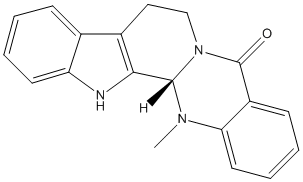
Chemical_Nomenclature : (1S)-21-methyl-3,13,21-triazapentacyclo[11.8.0.02,10.04,9.015,20]henicosa-2(10),4,6,8,15,17,19-heptaen-14-one
Canonical SMILES : CN1C2C3=C(CCN2C(=O)C4=CC=CC=C41)C5=CC=CC=C5N3
InChI : InChI=1S\/C19H17N3O\/c1-21-16-9-5-3-7-14(16)19(23)22-11-10-13-12-6-2-4-8-15(12)20-17(13)18(21)22\/h2-9,18,20H,10-11H2,1H3\/t18-\/m0\/s1
InChIKey : TXDUTHBFYKGSAH-SFHVURJKSA-N
Other name(s) : d-Evodiamine,(+)-Evodiamine,UNII-C01825BVNL,CHEBI:4948,CHEMBL463165,DTXSID10966123,ZINC898159
MW : 303.4
Formula : C19H17N3O
CAS_number : 518-17-2
PubChem : 442088
UniChem : TXDUTHBFYKGSAH-SFHVURJKSA-N
IUPHAR :
Wikipedia :
Target
References (6)
| Title : Protective effects of evodiamine in experimental paradigm of Alzheimer's disease - Wang_2018_Cogn.Neurodyn_12_303 |
| Author(s) : Wang D , Wang C , Liu L , Li S |
| Ref : Cogn Neurodyn , 12 :303 , 2018 |
| Abstract : Wang_2018_Cogn.Neurodyn_12_303 |
| ESTHER : Wang_2018_Cogn.Neurodyn_12_303 |
| PubMedSearch : Wang_2018_Cogn.Neurodyn_12_303 |
| PubMedID: 29765479 |
| Title : Pharmacological Basis for the Use of Evodiamine in Alzheimer's Disease: Antioxidation and Antiapoptosis - Zhang_2018_Int.J.Mol.Sci_19_ |
| Author(s) : Zhang Y , Wang J , Wang C , Li Z , Liu X , Zhang J , Lu J , Wang D |
| Ref : Int J Mol Sci , 19 : , 2018 |
| Abstract : Zhang_2018_Int.J.Mol.Sci_19_ |
| ESTHER : Zhang_2018_Int.J.Mol.Sci_19_ |
| PubMedSearch : Zhang_2018_Int.J.Mol.Sci_19_ |
| PubMedID: 29883380 |
| Title : Identification of a neuroprotective and selective butyrylcholinesterase inhibitor derived from the natural alkaloid evodiamine - Huang_2014_Eur.J.Med.Chem_81C_15 |
| Author(s) : Huang G , Kling B , Darras FH , Heilmann J , Decker M |
| Ref : Eur Journal of Medicinal Chemistry , 81C :15 , 2014 |
| Abstract : Huang_2014_Eur.J.Med.Chem_81C_15 |
| ESTHER : Huang_2014_Eur.J.Med.Chem_81C_15 |
| PubMedSearch : Huang_2014_Eur.J.Med.Chem_81C_15 |
| PubMedID: 24819955 |
| Title : Novel inhibitors of acetyl- and butyrylcholinesterase derived from the alkaloids dehydroevodiamine and rutaecarpine - Decker_2005_Eur.J.Med.Chem_40_305 |
| Author(s) : Decker M |
| Ref : Eur Journal of Medicinal Chemistry , 40 :305 , 2005 |
| Abstract : Decker_2005_Eur.J.Med.Chem_40_305 |
| ESTHER : Decker_2005_Eur.J.Med.Chem_40_305 |
| PubMedSearch : Decker_2005_Eur.J.Med.Chem_40_305 |
| PubMedID: 15725500 |
| Title : Dehydroevodiamine attenuates beta-amyloid peptide-induced amnesia in mice - Wang_2001_Eur.J.Pharmacol_413_221 |
| Author(s) : Wang HH , Chou CJ , Liao JF , Chen CF |
| Ref : European Journal of Pharmacology , 413 :221 , 2001 |
| Abstract : Wang_2001_Eur.J.Pharmacol_413_221 |
| ESTHER : Wang_2001_Eur.J.Pharmacol_413_221 |
| PubMedSearch : Wang_2001_Eur.J.Pharmacol_413_221 |
| PubMedID: 11226396 |
| Title : Novel anticholinesterase and antiamnesic activities of dehydroevodiamine, a constituent of Evodia rutaecarpa - Park_1996_Planta.Med_62_405 |
| Author(s) : Park CH , Kim SH , Choi W , Lee YJ , Kim JS , Kang SS , Suh YH |
| Ref : Planta Med , 62 :405 , 1996 |
| Abstract : Park_1996_Planta.Med_62_405 |
| ESTHER : Park_1996_Planta.Med_62_405 |
| PubMedSearch : Park_1996_Planta.Med_62_405 |
| PubMedID: 8923803 |