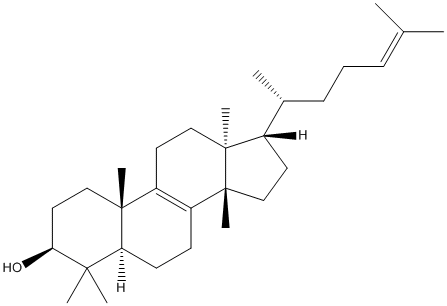
Euphol
King et al. found two naturally occurring terpendoids, pristimerin and euphol. Euphol less potent than pristimerin (IC50 = 315 +/- 1 nM) but inhibits also ABHD6 (IC50 = 98 +/- 8 nM)
General
Type : Terpenoid,Natural,Phenanthrene
Chemical_Nomenclature : (3S,5R,10S,13S,14S,17S)-4,4,10,13,14-pentamethyl-17-[(2R)-6-methylhept-5-en-2-yl]-2,3,5,6,7,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-3-ol
Canonical SMILES : CC(CCC=C(C)C)C1CCC2(C1(CCC3=C2CCC4C3(CCC(C4(C)C)O)C)C)C
InChI : InChI=1S\/C30H50O\/c1-20(2)10-9-11-21(3)22-14-18-30(8)24-12-13-25-27(4,5)26(31)16-17-28(25,6)23(24)15-19-29(22,30)7\/h10,21-22,25-26,31H,9,11-19H2,1-8H3\/t21-,22+,25+,26+,28-,29+,30-\/m1\/s1
InChIKey : CAHGCLMLTWQZNJ-WZLOIPHISA-N
Other name(s) : (+)-Euphol,Euphadienol,CHEBI:4940,(20R)-(+)-triucalla-8,24-diene-3beta-ol
MW : 426.71
Formula : C30H50O
CAS_number : 514-47-6
PubChem : 441678
UniChem : CAHGCLMLTWQZNJ-WZLOIPHISA-N
IUPHAR :
Wikipedia :
Target
Families : Euphol ligand of proteins in family: Monoglyceridelipase_lysophospholip || ABHD6-Lip
Stucture :
Protein :
References (2)
| Title : Monoglyceride lipase: Structure and inhibitors - Scalvini_2016_Chem.Phys.Lipids_197_13 |
| Author(s) : Scalvini L , Piomelli D , Mor M |
| Ref : Chemistry & Physic of Lipids , 197 :13 , 2016 |
| Abstract : Scalvini_2016_Chem.Phys.Lipids_197_13 |
| ESTHER : Scalvini_2016_Chem.Phys.Lipids_197_13 |
| PubMedSearch : Scalvini_2016_Chem.Phys.Lipids_197_13 |
| PubMedID: 26216043 |
| Title : Discovery of potent and reversible monoacylglycerol lipase inhibitors - King_2009_Chem.Biol_16_1045 |
| Author(s) : King AR , Dotsey EY , Lodola A , Jung KM , Ghomian A , Qiu Y , Fu J , Mor M , Piomelli D |
| Ref : Chemical Biology , 16 :1045 , 2009 |
| Abstract : King_2009_Chem.Biol_16_1045 |
| ESTHER : King_2009_Chem.Biol_16_1045 |
| PubMedSearch : King_2009_Chem.Biol_16_1045 |
| PubMedID: 19875078 |