Ebelactone-B
General
Type : Oxooxetan,Natural
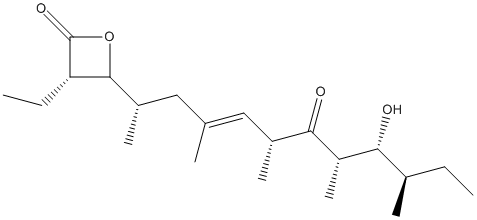
Chemical_Nomenclature : (3S,4S)-3-ethyl-4-[(E,2S,6R,8S,9R,10R)-9-hydroxy-4,6,8,10-tetramethyl-7-oxododec-4-en-2-yl]oxetan-2-one
Canonical SMILES : CCC1C(OC1=O)C(C)CC(=CC(C)C(=O)C(C)C(C(C)CC)O)C
InChI : InChI=1S\/C21H36O4\/c1-8-13(4)18(22)16(7)19(23)14(5)10-12(3)11-15(6)20-17(9-2)21(24)25-20\/h10,13-18,20,22H,8-9,11H2,1-7H3\/b12-10+\/t13-,14-,15+,16+,17+,18-,20+\/m1\/s1
InChIKey : UNBMQQNYLCPCHS-VYNDPHDASA-N*
Other name(s) : Ebelactone B,Ebelactone B microbial,NSC 335651,2-Oxetanone, 3-ethyl-4-(8-hydroxy-1,3,5,7,9-pentamethyl-6-oxo-3-undecenyl)-, (3S-(3-alpha,4-beta(1R*,3E,5S*,7R*,8S*,9S*)))-
MW : 352.50
Formula : C21H36O4
CAS_number : 76808-15-6
PubChem : 6436821
UniChem : UNBMQQNYLCPCHS-VYNDPHDASA-N*
IUPHAR :
Wikipedia :
Target
Families : Ebelactone-B ligand of proteins in family: Cutinase || Carboxypeptidase_S10 || Hepatic_Lipase || Lipoprotein_Lipase || Pancreatic_lipase
Stucture :
Protein :
References (8)
| Title : Novel beta-amino acid derivatives as inhibitors of cathepsin A. - Ruf_2012_J.Med.Chem_55_7636 |
| Author(s) : Ruf S , Buning C , Schreuder H , Horstick G , Linz W , Olpp T , Pernerstorfer J , Hiss K , Kroll K , Kannt A , Kohlmann M , Linz D , Hubschle T , Rutten H , Wirth K , Schmidt T , Sadowski T |
| Ref : Journal of Medicinal Chemistry , 55 :7636 , 2012 |
| Abstract : Ruf_2012_J.Med.Chem_55_7636 |
| ESTHER : Ruf_2012_J.Med.Chem_55_7636 |
| PubMedSearch : Ruf_2012_J.Med.Chem_55_7636 |
| PubMedID: 22861813 |
| Gene_locus related to this paper: human-CTSA |
| Title : A novel fluorogenic substrate for the measurement of endothelial lipase activity - Darrow_2011_J.Lipid.Res_52_374 |
| Author(s) : Darrow AL , Olson MW , Xin H , Burke SL , Smith C , Schalk-Hihi C , Williams R , Bayoumy SS , Deckman IC , Todd MJ , Damiano BP , Connelly MA |
| Ref : J Lipid Res , 52 :374 , 2011 |
| Abstract : Darrow_2011_J.Lipid.Res_52_374 |
| ESTHER : Darrow_2011_J.Lipid.Res_52_374 |
| PubMedSearch : Darrow_2011_J.Lipid.Res_52_374 |
| PubMedID: 21062953 |
| Gene_locus related to this paper: human-LIPG |
| Title : Crystal structure of a secreted lipase from Gibberella zeae reveals a novel double-lock mechanism - Lou_2010_Protein.Cell_1_760 |
| Author(s) : Lou Z , Li M , Sun Y , Liu Y , Liu Z , Wu W , Rao Z |
| Ref : Protein Cell , 1 :760 , 2010 |
| Abstract : Lou_2010_Protein.Cell_1_760 |
| ESTHER : Lou_2010_Protein.Cell_1_760 |
| PubMedSearch : Lou_2010_Protein.Cell_1_760 |
| PubMedID: 21203917 |
| Gene_locus related to this paper: gibze-q6wer3 |
| Title : Ebelactone B, an inhibitor of extracellular cathepsin A-type enzyme, suppresses platelet aggregation ex vivo in renovascular hypertensive rats - Ostrowska_2005_J.Cardiovasc.Pharmacol_45_348 |
| Author(s) : Ostrowska H , Kalinowska J , Chabielska E , Stankiewicz A , Kruszewski K , Buczko W |
| Ref : J Cardiovasc Pharmacol , 45 :348 , 2005 |
| Abstract : Ostrowska_2005_J.Cardiovasc.Pharmacol_45_348 |
| ESTHER : Ostrowska_2005_J.Cardiovasc.Pharmacol_45_348 |
| PubMedSearch : Ostrowska_2005_J.Cardiovasc.Pharmacol_45_348 |
| PubMedID: 15772524 |
| Title : Effects of ebelactone B, a lipase inhibitor, on intestinal fat absorption in the rat - Nonaka_1996_J.Enzyme.Inhib_10_57 |
| Author(s) : Nonaka Y , Ohtaki H , Ohtsuka E , Kocha T , Fukuda T , Takeuchi T , Aoyagi T |
| Ref : J Enzyme Inhib , 10 :57 , 1996 |
| Abstract : Nonaka_1996_J.Enzyme.Inhib_10_57 |
| ESTHER : Nonaka_1996_J.Enzyme.Inhib_10_57 |
| PubMedSearch : Nonaka_1996_J.Enzyme.Inhib_10_57 |
| PubMedID: 8835930 |
| Title : Degradation of bradykinin in human urine by carboxypeptidase Y-like exopeptidase and neutral endopeptidase and their inhibition by ebelactone B and phosphoramidon - Saito_1995_Int.J.Tissue.React_17_181 |
| Author(s) : Saito M , Majima M , Katori M , Sanjou Y , Suyama I , Shiokawa H , Koshiba K , Aoyagi T |
| Ref : Int J Tissue React , 17 :181 , 1995 |
| Abstract : Saito_1995_Int.J.Tissue.React_17_181 |
| ESTHER : Saito_1995_Int.J.Tissue.React_17_181 |
| PubMedSearch : Saito_1995_Int.J.Tissue.React_17_181 |
| PubMedID: 8835628 |
| Title : Ebelactone, an inhibitor of esterase, produced by actinomycetes - |
| Author(s) : Umezawa H , Aoyagi T , Uotani K , Hamada M , Takeuchi T , Takahashi S |
| Ref : J Antibiot (Tokyo) , 33 :1594 , 1980 |
| PubMedID: 7251497 |