ENA-713~Rivastigmine
General
Type : Derivative of Rivastigmine,Carbamate,Drug
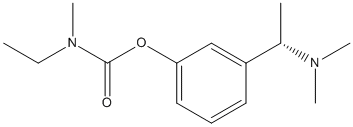
Chemical_Nomenclature : S-N-ethyl-3-[(1-dimethyl-amino)ethyl]-N-methyl-phenylcarbamate hydrogentartrate
Canonical SMILES : CCN(C)C(=O)OC1=CC=CC(=C1)C(C)N(C)C
InChI : InChI=1S\/C14H22N2O2\/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4\/h7-11H,6H2,1-5H3 || InChI=1S\/C14H22N2O2\/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4\/h7-11H,6H2,1-5H3\/t11-\/m0\/s1
InChIKey : XSVMFMHYUFZWBK-UHFFFAOYSA-N || XSVMFMHYUFZWBK-NSHDSACASA-N
Other name(s) : carbamoylatine,Exelon,ENA-713,Rivastigmine,SDZ-212-713,SDZ-ENA-713
MW : 250.34
Formula : C14H22N2O2
CAS_number : 123441-03-2 || 129101-54-8
UniChem : XSVMFMHYUFZWBK-UHFFFAOYSA-N || XSVMFMHYUFZWBK-NSHDSACASA-N
IUPHAR : 6602
Wikipedia : Rivastigmine
Target
References (12)
| Title : Inhibition of human carboxylesterases hCE1 and hiCE by cholinesterase inhibitors - Tsurkan_2013_Chem.Biol.Interact_203_226 |
| Author(s) : Tsurkan LG , Hatfield MJ , Edwards CC , Hyatt JL , Potter PM |
| Ref : Chemico-Biological Interactions , 203 :226 , 2013 |
| Abstract : Tsurkan_2013_Chem.Biol.Interact_203_226 |
| ESTHER : Tsurkan_2013_Chem.Biol.Interact_203_226 |
| PubMedSearch : Tsurkan_2013_Chem.Biol.Interact_203_226 |
| PubMedID: 23123248 |
| Title : Kinetic and structural studies on the interaction of cholinesterases with the anti-Alzheimer drug rivastigmine - Bar-On_2002_Biochemistry_41_3555 |
| Author(s) : Bar-On P , Millard CB , Harel M , Dvir H , Enz A , Sussman JL , Silman I |
| Ref : Biochemistry , 41 :3555 , 2002 |
| Abstract : Bar-On_2002_Biochemistry_41_3555 |
| ESTHER : Bar-On_2002_Biochemistry_41_3555 |
| PubMedSearch : Bar-On_2002_Biochemistry_41_3555 |
| PubMedID: 11888271 |
| Gene_locus related to this paper: torca-ACHE |
| Title : Alzheimer's disease: seeking new ways to preserve brain function. Interview by Alice V. Luddington - Davis_1999_Geriatrics_54_42 |
| Author(s) : Davis KL |
| Ref : Geriatrics , 54 :42 , 1999 |
| Abstract : Davis_1999_Geriatrics_54_42 |
| ESTHER : Davis_1999_Geriatrics_54_42 |
| PubMedSearch : Davis_1999_Geriatrics_54_42 |
| PubMedID: 10024872 |
| Title : Invited review: Cholinesterase inhibitors for Alzheimer's disease therapy: from tacrine to future applications - Giacobini_1998_Neurochem.Int_32_413 |
| Author(s) : Giacobini E |
| Ref : Neurochem Int , 32 :413 , 1998 |
| Abstract : Giacobini_1998_Neurochem.Int_32_413 |
| ESTHER : Giacobini_1998_Neurochem.Int_32_413 |
| PubMedSearch : Giacobini_1998_Neurochem.Int_32_413 |
| PubMedID: 9676739 |
| Title : Cerebro-protective effects of ENA713, a novel acetylcholinesterase inhibitor, in closed head injury in the rat - Chen_1998_Brain.Res_784_18 |
| Author(s) : Chen Y , Shohami E , Bass R , Weinstock M |
| Ref : Brain Research , 784 :18 , 1998 |
| Abstract : Chen_1998_Brain.Res_784_18 |
| ESTHER : Chen_1998_Brain.Res_784_18 |
| PubMedSearch : Chen_1998_Brain.Res_784_18 |
| PubMedID: 9518537 |
| Title : Rivastigmine, a brain-selective acetylcholinesterase inhibitor, ameliorates cognitive and motor deficits induced by closed-head injury in the mouse - Chen_1998_J.Neurotrauma_15_231 |
| Author(s) : Chen Y , Shohami E , Constantini S , Weinstock M |
| Ref : Journal of Neurotrauma , 15 :231 , 1998 |
| Abstract : Chen_1998_J.Neurotrauma_15_231 |
| ESTHER : Chen_1998_J.Neurotrauma_15_231 |
| PubMedSearch : Chen_1998_J.Neurotrauma_15_231 |
| PubMedID: 9555969 |
| Title : Dose-dependent CSF acetylcholinesterase inhibition by SDZ ENA 713 in Alzheimer's disease - Cutler_1998_Acta.Neurol.Scand_97_244 |
| Author(s) : Cutler NR , Polinsky RJ , Sramek JJ , Enz A , Jhee SS , Mancione L , Hourani J , Zolnouni P |
| Ref : Acta Neurologica Scandinavica , 97 :244 , 1998 |
| Abstract : Cutler_1998_Acta.Neurol.Scand_97_244 |
| ESTHER : Cutler_1998_Acta.Neurol.Scand_97_244 |
| PubMedSearch : Cutler_1998_Acta.Neurol.Scand_97_244 |
| PubMedID: 9576639 |
| Title : Disposition of SDZ ENA 713, an acetylcholinesterase inhibitor, in the rabbit - Habucky_1998_Biopharm.Drug.Dispos_19_285 |
| Author(s) : Habucky K , Tse FL |
| Ref : Biopharmaceutics & Drug Disposition , 19 :285 , 1998 |
| Abstract : Habucky_1998_Biopharm.Drug.Dispos_19_285 |
| ESTHER : Habucky_1998_Biopharm.Drug.Dispos_19_285 |
| PubMedSearch : Habucky_1998_Biopharm.Drug.Dispos_19_285 |
| PubMedID: 9673779 |
| Title : Clinical pharmacology of rivastigmine: a new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer's disease - Polinsky_1998_Clin.Ther_20_634 |
| Author(s) : Polinsky RJ |
| Ref : Clin Ther , 20 :634 , 1998 |
| Abstract : Polinsky_1998_Clin.Ther_20_634 |
| ESTHER : Polinsky_1998_Clin.Ther_20_634 |
| PubMedSearch : Polinsky_1998_Clin.Ther_20_634 |
| PubMedID: 9737824 |
| Title : Treating Alzheimer's disease.Pharmacologic options now and in the near future - Tariot_1997_Postgrad.Med_101_73 |
| Author(s) : Tariot PN , Schneider L , Porsteinsson AP |
| Ref : Postgrad Med , 101 :73 , 1997 |
| Abstract : Tariot_1997_Postgrad.Med_101_73 |
| ESTHER : Tariot_1997_Postgrad.Med_101_73 |
| PubMedSearch : Tariot_1997_Postgrad.Med_101_73 |
| PubMedID: 9194867 |
| Title : Inhibition of acetylcholinesterase modulates the autoregulation of cerebral blood flow and attenuates ischemic brain metabolism in hypertensive rats - Sadoshima_1995_J.Cerebral.Blood.Flow.Metab_15_845 |
| Author(s) : Sadoshima S , Ibayashi S , Fujii K , Nagao T , Sugimori H , Fujishima M |
| Ref : Journal of Cerebral Blood Flow & Metabolism , 15 :845 , 1995 |
| Abstract : Sadoshima_1995_J.Cerebral.Blood.Flow.Metab_15_845 |
| ESTHER : Sadoshima_1995_J.Cerebral.Blood.Flow.Metab_15_845 |
| PubMedSearch : Sadoshima_1995_J.Cerebral.Blood.Flow.Metab_15_845 |
| PubMedID: 7673377 |
| Title : Post-ischemic administration of the acetylcholinesterase inhibitor ENA-713 prevents delayed neuronal death in the gerbil hippocampus - Tanaka_1995_Neurochem.Res_20_663 |
| Author(s) : Tanaka K , Mizukawa K , Ogawa N , Mori A |
| Ref : Neurochemical Research , 20 :663 , 1995 |
| Abstract : Tanaka_1995_Neurochem.Res_20_663 |
| ESTHER : Tanaka_1995_Neurochem.Res_20_663 |
| PubMedSearch : Tanaka_1995_Neurochem.Res_20_663 |
| PubMedID: 756636 |