Diuron
Diuron is an algicide and herbicide of the phenylurea class that inhibits photosynthesis, a photosystem-II inhibitor, a xenobiotic, an environmental contaminant and a mitochondrial respiratory-chain inhibitor
General
Type : Herbicide,Not A\/B H target,Urea derivative
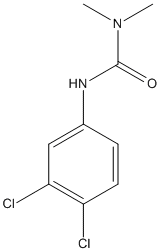
Chemical_Nomenclature : 3-(3,4-dichlorophenyl)-1,1-dimethylurea
Canonical SMILES : CN(C)C(=O)NC1=CC(=C(C=C1)Cl)Cl
InChI : InChI=1S\/C9H10Cl2N2O\/c1-13(2)9(14)12-6-3-4-7(10)8(11)5-6\/h3-5H,1-2H3,(H,12,14)
InChIKey : XMTQQYYKAHVGBJ-UHFFFAOYSA-N
Other name(s) : 3-(3,4-Dichlorophenyl)-1,1-dimethylurea,DCMU,Duran,Dichlorfenidim
MW : 233.09
Formula : C9H10Cl2N2O
CAS_number : 330-54-1
PubChem : 3120
UniChem : XMTQQYYKAHVGBJ-UHFFFAOYSA-N
IUPHAR :
Wikipedia : DCMU
Target
Families : Diuron ligand of proteins in family: ACHE || Epoxide_hydrolase
Stucture : 4C4X Crystal structure of human bifunctional epoxide hydroxylase 2 complexed with C9
Protein : human-EPHX2
References (5)
| Title : A combination of spin diffusion methods for the determination of protein-ligand complex structural ensembles - Pilger_2015_Angew.Chem.Int.Ed.Engl_54_6511 |
| Author(s) : Pilger J , Mazur A , Monecke P , Schreuder H , Elshorst B , Bartoschek S , Langer T , Schiffer A , Krimm I , Wegstroth M , Lee D , Hessler G , Wendt KU , Becker S , Griesinger C |
| Ref : Angew Chem Int Ed Engl , 54 :6511 , 2015 |
| Abstract : Pilger_2015_Angew.Chem.Int.Ed.Engl_54_6511 |
| ESTHER : Pilger_2015_Angew.Chem.Int.Ed.Engl_54_6511 |
| PubMedSearch : Pilger_2015_Angew.Chem.Int.Ed.Engl_54_6511 |
| PubMedID: 25877959 |
| Gene_locus related to this paper: human-EPHX2 |
| Title : Evolutionary expansion of the amidohydrolase superfamily in bacteria in response to the synthetic compounds molinate and diuron - Sugrue_2015_Appl.Environ.Microbiol_81_2612 |
| Author(s) : Sugrue E , Fraser NJ , Hopkins DH , Carr PD , Khurana JL , Oakeshott JG , Scott C , Jackson CJ |
| Ref : Applied Environmental Microbiology , 81 :2612 , 2015 |
| Abstract : Sugrue_2015_Appl.Environ.Microbiol_81_2612 |
| ESTHER : Sugrue_2015_Appl.Environ.Microbiol_81_2612 |
| PubMedSearch : Sugrue_2015_Appl.Environ.Microbiol_81_2612 |
| PubMedID: 25636851 |
| Title : Acetylcholinesterase inhibitors: pharmacology and toxicology - Colovic_2013_Curr.Neuropharmacol_11_315 |
| Author(s) : Colovic MB , Krstic DZ , Lazarevic-Pasti TD , Bondzic AM , Vasic VM |
| Ref : Curr Neuropharmacol , 11 :315 , 2013 |
| Abstract : Colovic_2013_Curr.Neuropharmacol_11_315 |
| ESTHER : Colovic_2013_Curr.Neuropharmacol_11_315 |
| PubMedSearch : Colovic_2013_Curr.Neuropharmacol_11_315 |
| PubMedID: 24179466 |
| Title : Toxicological effect of herbicides (diuron and bentazon) on snake venom and electric eel acetylcholinesterase - Ahmed_2012_Bull.Environ.Contam.Toxicol_89_229 |
| Author(s) : Ahmed M , Latif N , Khan RA , Ahmad A |
| Ref : Bulletin of Environmental Contamination & Toxicology , 89 :229 , 2012 |
| Abstract : Ahmed_2012_Bull.Environ.Contam.Toxicol_89_229 |
| ESTHER : Ahmed_2012_Bull.Environ.Contam.Toxicol_89_229 |
| PubMedSearch : Ahmed_2012_Bull.Environ.Contam.Toxicol_89_229 |
| PubMedID: 22653306 |
| Title : Effects of carbofuran, diuron, and nicosulfuron on acetylcholinesterase activity in goldfish (Carassius auratus) - Bretaud_2000_Ecotoxicol.Environ.Saf_47_117 |
| Author(s) : Bretaud S , Toutant JP , Saglio P |
| Ref : Ecotoxicology & Environmental Safety , 47 :117 , 2000 |
| Abstract : Bretaud_2000_Ecotoxicol.Environ.Saf_47_117 |
| ESTHER : Bretaud_2000_Ecotoxicol.Environ.Saf_47_117 |
| PubMedSearch : Bretaud_2000_Ecotoxicol.Environ.Saf_47_117 |
| PubMedID: 11023689 |