Diprotin-A
CID: 137319704: Diprotin A (TFA); Diprotin A TFA; 209248-71-5; (2S,3S)-2-[[(2S)-1-[(2S,3S)-2-amino-3-methylpentanoyl]pyrrolidine-2-carbonyl]amino]-3-methylpentanoic acid;2,2,2-trifluoroacetic acid; Ile-Pro-Pro (TFA); HY-111174A; Diprotin A TFA (Ile-Pro-Pro (TFA)); CS-0077835
General
Type : Peptide,Pyrrolidine
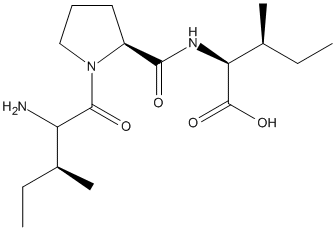
Chemical_Nomenclature : (2S,3S)-2-[[(2S)-1-[(2S,3S)-2-amino-3-methylpentanoyl]pyrrolidine-2-carbonyl]amino]-3-methylpentanoic acid
Canonical SMILES : CCC(C)C(C(=O)N1CCCC1C(=O)NC(C(C)CC)C(=O)O)N
InChI : InChI=1S\/C17H31N3O4\/c1-5-10(3)13(18)16(22)20-9-7-8-12(20)15(21)19-14(17(23)24)11(4)6-2\/h10-14H,5-9,18H2,1-4H3,(H,19,21)(H,23,24)\/t10-,11-,12-,13-,14-\/m0\/s1
InChIKey : JNTMAZFVYNDPLB-UHFFFAOYSA-N
Other name(s) : Diprotin A,Ile-Pro-Ile,NSC602337,Isoleucylprolylisoleucine,L-isoleucyl-l-prolyl-l-isoleucine,HIV inhibitor,AC1Q5KXE,CHEMBL214381,C17H31N3O4
MW : 341.44
Formula : C17H31N3O4
CAS_number : 90614-48-5
PubChem : 94701
UniChem : JNTMAZFVYNDPLB-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : Diprotin-A ligand of proteins in family: DPP4N_Peptidase_S9
Stucture :
Protein : human-DPP4
References (4)
| Title : Diprotin A TFA Exerts Neurovascular Protection in Ischemic Cerebral Stroke - Zhou_2022_Front.Neurosci_16_861059 |
| Author(s) : Zhou MY , Zhang YJ , Ding HM , Wu WF , Cai WW , Wang YQ , Geng DQ |
| Ref : Front Neurosci , 16 :861059 , 2022 |
| Abstract : Zhou_2022_Front.Neurosci_16_861059 |
| ESTHER : Zhou_2022_Front.Neurosci_16_861059 |
| PubMedSearch : Zhou_2022_Front.Neurosci_16_861059 |
| PubMedID: 35615279 |
| Title : Crystal structures of human dipeptidyl peptidase IV in its apo and diprotin B-complexed forms - Hiramatsu_2007_Acta.Biochim.Biophys.Sin.(Shanghai)_39_335 |
| Author(s) : Hiramatsu H , Kyono K , Yamamoto A , Saeki K , Shima H , Sugiyama S , Inaka K , Shimizu R |
| Ref : Acta Biochim Biophys Sin (Shanghai) , 39 :335 , 2007 |
| Abstract : Hiramatsu_2007_Acta.Biochim.Biophys.Sin.(Shanghai)_39_335 |
| ESTHER : Hiramatsu_2007_Acta.Biochim.Biophys.Sin.(Shanghai)_39_335 |
| PubMedSearch : Hiramatsu_2007_Acta.Biochim.Biophys.Sin.(Shanghai)_39_335 |
| PubMedID: 17492130 |
| Gene_locus related to this paper: human-DPP4 |
| Title : The crystal structure of human dipeptidyl peptidase IV (DPPIV) complex with diprotin A - Hiramatsu_2004_Biol.Chem_385_561 |
| Author(s) : Hiramatsu H , Yamamoto A , Kyono K , Higashiyama Y , Fukushima C , Shima H , Sugiyama S , Inaka K , Shimizu R |
| Ref : Biol Chem , 385 :561 , 2004 |
| Abstract : Hiramatsu_2004_Biol.Chem_385_561 |
| ESTHER : Hiramatsu_2004_Biol.Chem_385_561 |
| PubMedSearch : Hiramatsu_2004_Biol.Chem_385_561 |
| PubMedID: 15255191 |
| Gene_locus related to this paper: human-DPP4 |
| Title : Structural basis of proline-specific exopeptidase activity as observed in human dipeptidyl peptidase-IV - Thoma_2003_Structure_11_947 |
| Author(s) : Thoma R , Loffler B , Stihle M , Huber W , Ruf A , Hennig M |
| Ref : Structure , 11 :947 , 2003 |
| Abstract : Thoma_2003_Structure_11_947 |
| ESTHER : Thoma_2003_Structure_11_947 |
| PubMedSearch : Thoma_2003_Structure_11_947 |
| PubMedID: 12906826 |
| Gene_locus related to this paper: human-DPP4 |