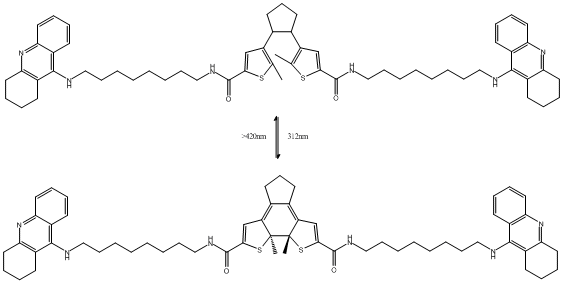
DTE-bis8-Tacrine
General
Type : Derivative of Tacrine,Alkyl linked bis-ligand,Acridine,Photoswitch,Sulfur Compound
Chemical_Nomenclature :
Canonical SMILES : C12=C7[C@@]([C@@]3(C(=C1CCC2)C=C(S3)C(=O)NCCCCCCCCNC4=C6C(=NC5=C4C=CC=C5)CCCC6)C)(SC(=C7)C(NCCCCCCCCNC8=C\%10C(=NC9=C8CCCC9)C=CC=C\%10)=O)C || C1(C(CCC1)C2=C(SC(=C2)C(=O)NCCCCCCCCNC3=C5C(=NC4=C3C=CC=C4)CCCC5)C)C6=C(SC(=C6)C(NCCCCCCCCNC7=C9C(=NC8=C7CCCC8)C=CC=C9)=O)C
InChI : InChI=1S\/C59H74N6O2S2\/c1-58-46(38-52(68-58)56(66)62-36-21-9-5-3-7-19-34-60-54-42-24-11-15-30-48(42)64-49-31-16-12-25-43(49)54)40-28-23-29-41(40)47-39-53(69-59(47,58)2)57(67)63-37-22-10-6-4-8-20-35-61-55-44-26-13-17-32-50(44)65-51-33-18-14-27-45(51)55\/h11,13,15,17,24,26,30,32,38-39H,3-10,12,14,16,18-23,25,27-29,31,33-37H2,1-2H3,(H,60,64)(H,61,65)(H,62,66)(H,63,67)\/t58-,59-\/m0\/s1 || InChI=1S\/C59H76N6O2S2\/c1-40-48(38-54(68-40)58(66)62-36-21-9-5-3-7-19-34-60-56-44-24-11-15-30-50(44)64-51-31-16-12-25-45(51)56)42-28-23-29-43(42)49-39-55(69-41(49)2)59(67)63-37-22-10-6-4-8-20-35-61-57-46-26-13-17-32-52(46)65-53-33-18-14-27-47(53)57\/h11,13,15,17,24,26,30,32,38-39,42-43H,3-10,12,14,16,18-23,25,27-29,31,33-37H2,1-2H3,(H,60,64)(H,61,65)(H,62,66)(H,63,67)
InChIKey : OSVHTUAJGFGBMA-LYJOYROPSA-N || CWWXOTNZFOZOLJ-UHFFFAOYSA-N
Other name(s) :
MW : 963.39 || 965.40
Formula : C59H74N6O2S2 || C59H76N6O2S2
CAS_number :
PubChem :
UniChem : OSVHTUAJGFGBMA-LYJOYROPSA-N || CWWXOTNZFOZOLJ-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (2)
| Title : Photopharmacology on Acetylcholinesterase: Novel Photoswitchable Inhibitors with Improved Pharmacological Profiles - Scheiner_2021_ChemPhotoChem_5_149 |
| Author(s) : Scheiner M , Sink A , Spatz P , Endres E , Decker M |
| Ref : ChemPhotoChem , 5 :149 , 2021 |
| Abstract : Scheiner_2021_ChemPhotoChem_5_149 |
| ESTHER : Scheiner_2021_ChemPhotoChem_5_149 |
| PubMedSearch : Scheiner_2021_ChemPhotoChem_5_149 |
| PubMedID: |
| Title : Acetylcholinesterase inhibitors with photoswitchable inhibition of beta-amyloid aggregation - Chen_2014_ACS.Chem.Neurosci_5_377 |
| Author(s) : Chen X , Wehle S , Kuzmanovic N , Merget B , Holzgrabe U , Konig B , Sotriffer CA , Decker M |
| Ref : ACS Chem Neurosci , 5 :377 , 2014 |
| Abstract : Chen_2014_ACS.Chem.Neurosci_5_377 |
| ESTHER : Chen_2014_ACS.Chem.Neurosci_5_377 |
| PubMedSearch : Chen_2014_ACS.Chem.Neurosci_5_377 |
| PubMedID: 24628027 |