Cyclipostin
Ten inhibitors of HSL, termed cyclipostins, have been isolated from the mycelium of Streptomyces species DSM 13381. Phosphate esters of long chain lipophilic alcohols. All members of the Cyclipostins family are very potent inhibitors of hormone-sensitive lipase (HSL) a therapeutic target for type II diabetes. The different Cyclopostins are: Cyclipostin P; CID 101182228; LXXPVFWDVXTYTB-BPADPFCFSA-N; (1beta)-4beta-(Hexadecyloxy)-6-methyl-8-oxo-3,5,9-trioxa-4-phosphabicyclo[5.3.0]deca-6-ene 4-oxide; (6S)-6alpha-(Hexadecyloxy)-8-methyl-3,3abeta-dihydro-1H,4H,6H-2,5,7-trioxa-6-phosphaazulene-1-one 6-oxide --- Cyclipostin P2; Cyclipostin P 2; IGWOOZHLAHUMBM-BPADPFCFSA-N; CID 101182229 --- Cyclipostin T; CID 101182233; UEXFAQDUVJPKAX-GFTAOQHMSA-N --- Cyclipostin S; CID 101182232; NESUOKQUVWDIJP-UBIBJYAGSA-N --- Cyclipostin R; CID 101182230; BHKGHKHKDXVQJF-CUXXENAFSA-N --- Cyclipostin N; CID 101182227; YNKNWOLTEQUDMB-OJQMSQGESA-N --- Cyclipostin F; CID 101182226; AEULNKPXALBIHK-DXHYANOHSA-N --- Cyclipostin A; CID 101182225; JGTUCPMGHURLJI-FRMQRXHYSA-N --- Cyclipostin T2; CID 101182234; SYOULUNKPBFPTF-GFTAOQHMSA-N --- trans-Cyclipostin P; CID 101805205; LXXPVFWDVXTYTB-UHWFRNQFSA-N
General
Type : Organophosphate,Cyclic OP
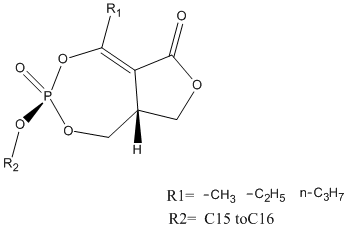
Chemical_Nomenclature : (8aR)-3-hexadecoxy-5-methyl-3-oxido-8,8a-dihydro-1H-furo[3,4-e][1,3,2]dioxaphosphepin-3-ium-6-one
Canonical SMILES :
InChI : InChI=1S\/C23H41O6P\/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-27-30(25)28-19-21-18-26-23(24)22(21)20(2)29-30\/h21H,3-19H2,1-2H3\/t21-,30?\/m1\/s1
InChIKey : LXXPVFWDVXTYTB-BPADPFCFSA-N
Other name(s) : (3aR,6R)-3-R1-5-R2-3-oxo-8,8a-dihydro-1H-furo[3,4-e][1,3,2]dioxaphosphepin-6-one
MW :
Formula :
CAS_number :
PubChem : 101182228
UniChem : LXXPVFWDVXTYTB-BPADPFCFSA-N
IUPHAR :
Wikipedia :
Target
Families : Cyclipostin ligand of proteins in family: Hormone-sensitive_lipase_like
Stucture :
Protein :
References (4)
| Title : Cyclophostin and Cyclipostins analogues, new promising molecules to treat mycobacterial-related diseases - Nguyen_2018_Int.J.Antimicrob.Agents_51_651 |
| Author(s) : Nguyen PC , Madani A , Santucci P , Martin BP , Paudel RR , Delattre S , Herrmann JL , Spilling CD , Kremer L , Canaan S , Cavalier JF |
| Ref : Int J Antimicrob Agents , 51 :651 , 2018 |
| Abstract : Nguyen_2018_Int.J.Antimicrob.Agents_51_651 |
| ESTHER : Nguyen_2018_Int.J.Antimicrob.Agents_51_651 |
| PubMedSearch : Nguyen_2018_Int.J.Antimicrob.Agents_51_651 |
| PubMedID: 29241819 |
| Title : Rat hormone sensitive lipase inhibition by cyclipostins and their analogs - Vasilieva_2015_Bioorg.Med.Chem_23_944 |
| Author(s) : Vasilieva E , Dutta S , Malla RK , Martin BP , Spilling CD , Dupureur CM |
| Ref : Bioorganic & Medicinal Chemistry , 23 :944 , 2015 |
| Abstract : Vasilieva_2015_Bioorg.Med.Chem_23_944 |
| ESTHER : Vasilieva_2015_Bioorg.Med.Chem_23_944 |
| PubMedSearch : Vasilieva_2015_Bioorg.Med.Chem_23_944 |
| PubMedID: 25678014 |
| Gene_locus related to this paper: ratno-hslip |
| Title : The first total synthesis of (+\/-)-cyclophostin and (+\/-)-cyclipostin P: inhibitors of the serine hydrolases acetyl cholinesterase and hormone sensitive lipase - Malla_2011_Org.Lett_13_3094 |
| Author(s) : Malla RK , Bandyopadhyay S , Spilling CD , Dutta S , Dupureur CM |
| Ref : Org Lett , 13 :3094 , 2011 |
| Abstract : Malla_2011_Org.Lett_13_3094 |
| ESTHER : Malla_2011_Org.Lett_13_3094 |
| PubMedSearch : Malla_2011_Org.Lett_13_3094 |
| PubMedID: 21591624 |
| Title : Cyclipostins, novel hormone-sensitive lipase inhibitors from Streptomyces sp. DSM 13381. II. Isolation, structure elucidation and biological properties - Vertesy_2002_J.Antibiot.(Tokyo)_55_480 |
| Author(s) : Vertesy L , Beck B , Bronstrup M , Ehrlich K , Kurz M , Muller G , Schummer D , Seibert G |
| Ref : J Antibiot (Tokyo) , 55 :480 , 2002 |
| Abstract : Vertesy_2002_J.Antibiot.(Tokyo)_55_480 |
| ESTHER : Vertesy_2002_J.Antibiot.(Tokyo)_55_480 |
| PubMedSearch : Vertesy_2002_J.Antibiot.(Tokyo)_55_480 |
| PubMedID: 12139017 |