Cyanophos
Cyanophos is a yellow to reddish-yellow transparent liquid. Used as an insecticide against rice stem borers and house flies. Not registered as a pesticide in the U.S. (EPA, 1998)
General
Type : Organophosphate,Cyanide,Insecticide,Sulfur Compound
Chemical_Nomenclature : 4-dimethoxyphosphinothioyloxybenzonitrile
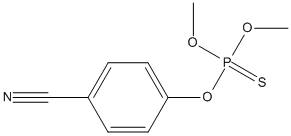
Canonical SMILES : COP(=S)(OC)OC1=CC=C(C=C1)C#N
InChI : InChI=1S\/C9H10NO3PS\/c1-11-14(15,12-2)13-9-5-3-8(7-10)4-6-9\/h3-6H,1-2H3
InChIKey : SCKHCCSZFPSHGR-UHFFFAOYSA-N
Other name(s) : Ciafos,Cyanox,CYAP,Cynock
MW : 243.21
Formula : C9H10NO3PS
CAS_number : 2636-26-2
PubChem : 17522
UniChem : SCKHCCSZFPSHGR-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (4)
| Title : Enhancing agents for phytoremediation of soil contaminated by cyanophos - Ali Romeh_2015_Ecotoxicol.Environ.Saf_117_124 |
| Author(s) : Ali Romeh A |
| Ref : Ecotoxicology & Environmental Safety , 117 :124 , 2015 |
| Abstract : Ali Romeh_2015_Ecotoxicol.Environ.Saf_117_124 |
| ESTHER : Ali Romeh_2015_Ecotoxicol.Environ.Saf_117_124 |
| PubMedSearch : Ali Romeh_2015_Ecotoxicol.Environ.Saf_117_124 |
| PubMedID: 25847752 |
| Title : Rapid simultaneous determination for organophosphorus pesticides in human serum by LC-MS - Inoue_2007_J.Pharm.Biomed.Anal_44_258 |
| Author(s) : Inoue S , Saito T , Mase H , Suzuki Y , Takazawa K , Yamamoto I , Inokuchi S |
| Ref : J Pharm Biomed Anal , 44 :258 , 2007 |
| Abstract : Inoue_2007_J.Pharm.Biomed.Anal_44_258 |
| ESTHER : Inoue_2007_J.Pharm.Biomed.Anal_44_258 |
| PubMedSearch : Inoue_2007_J.Pharm.Biomed.Anal_44_258 |
| PubMedID: 17337150 |
| Title : The stability of organophosphorus insecticides in fresh blood - Ageda_2006_Leg.Med.(Tokyo)_8_144 |
| Author(s) : Ageda S , Fuke C , Ihama Y , Miyazaki T |
| Ref : Leg Med (Tokyo) , 8 :144 , 2006 |
| Abstract : Ageda_2006_Leg.Med.(Tokyo)_8_144 |
| ESTHER : Ageda_2006_Leg.Med.(Tokyo)_8_144 |
| PubMedSearch : Ageda_2006_Leg.Med.(Tokyo)_8_144 |
| PubMedID: 16517205 |
| Title : Oxidative Activation and Degradation of Organophosphorus Pesticides Mediated by Iron Porphyrins - Fujisawa_2005_J.Pestic.Sci_30_103 |
| Author(s) : Fujisawa T , Katagi T |
| Ref : Journal of Pesticide Science , 30 :103 , 2005 |
| Abstract : Fujisawa_2005_J.Pestic.Sci_30_103 |
| ESTHER : Fujisawa_2005_J.Pestic.Sci_30_103 |
| PubMedSearch : Fujisawa_2005_J.Pestic.Sci_30_103 |
| PubMedID: |