Croneton
General
Type : Carbamate,Insecticide,Sulfur Compound
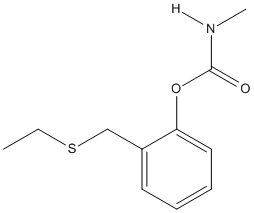
Chemical_Nomenclature : [2-(ethylsulfanylmethyl)phenyl] N-methylcarbamate
Canonical SMILES : CCSCC1=CC=CC=C1OC(=O)NC
InChI : InChI=1S\/C11H15NO2S\/c1-3-15-8-9-6-4-5-7-10(9)14-11(13)12-2\/h4-7H,3,8H2,1-2H3,(H,12,13)
InChIKey : HEZNVIYQEUHLNI-UHFFFAOYSA-N
Other name(s) : Ethiofencarb,Arylmate,Kronetone,Croneton 500,CHEBI:38483
MW : 225.30
Formula : C11H15NO2S
CAS_number : 29973-13-5
PubChem : 34766
UniChem : HEZNVIYQEUHLNI-UHFFFAOYSA-N
IUPHAR :
Wikipedia : Ethiofencarb
Target
References (4)
| Title : Lethal poisoning with ethiofencarb and ethanol - Al-Samarraie_2009_J.Anal.Toxicol_33_389 |
| Author(s) : Al-Samarraie MS , Karinen R , Rognum T , Hasvold I , Opdal Stokke M , Christophersen AS |
| Ref : J Anal Toxicol , 33 :389 , 2009 |
| Abstract : Al-Samarraie_2009_J.Anal.Toxicol_33_389 |
| ESTHER : Al-Samarraie_2009_J.Anal.Toxicol_33_389 |
| PubMedSearch : Al-Samarraie_2009_J.Anal.Toxicol_33_389 |
| PubMedID: 19796510 |
| Title : Extrahepatic metabolism of carbamate and organophosphate thioether compounds by the flavin-containing monooxygenase and cytochrome P450 systems - Furnes_2005_Drug.Metab.Dispos_33_214 |
| Author(s) : Furnes B , Schlenk D |
| Ref : Drug Metabolism & Disposition: The Biological Fate of Chemicals , 33 :214 , 2005 |
| Abstract : Furnes_2005_Drug.Metab.Dispos_33_214 |
| ESTHER : Furnes_2005_Drug.Metab.Dispos_33_214 |
| PubMedSearch : Furnes_2005_Drug.Metab.Dispos_33_214 |
| PubMedID: 15547051 |
| Title : Kinetic Study of the Degradation of Ethiofencarb in Aqueous Solutions - Sanz-Asensio_1997_Pest.Sci_50_187 |
| Author(s) : Sanz-Asensio J , Plaza-Medina M , Martinez-Soria MT |
| Ref : Pest Sci , 50 :187 , 1997 |
| Abstract : Sanz-Asensio_1997_Pest.Sci_50_187 |
| ESTHER : Sanz-Asensio_1997_Pest.Sci_50_187 |
| PubMedSearch : Sanz-Asensio_1997_Pest.Sci_50_187 |
| PubMedID: |
| Title : Poster: Anticholinesterase potency of Hostaquick, Pirimor, Croneton and Methomyl for Aphids and Entomophages - |
| Author(s) : Sazonova IN , Novozhilov KV , Nikanorova EV , Shvets EK |
| Ref : In: Cholinesterases: Structure, Function, Mechanism, Genetics, and Cell Biology , (Massoulie J, Barnard EA, Chatonnet A, Bacou F, Doctor BP, Quinn DM) American Chemical Society, Washington, DC :277 , 1991 |
| PubMedID: |