Cotinine
Cotinine is the major metabolite of nicotine. It is an alkaloid commonly found in Nicotiana tabacum. The N-glucuronide conjugate of cotinine is a major urinary metabolite of Nicotine. It thus serves as a biomarker of exposure to tobacco smoking. It has CNS stimulating properties
General
Type : Derivative of Nicotine,Pyrrolidine,Natural,Alkaloid,Drug,Nicotinic agonist,Not A\/B H target
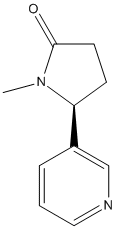
Chemical_Nomenclature : (5S)-1-methyl-5-pyridin-3-ylpyrrolidin-2-one
Canonical SMILES : CN1C(CCC1=O)C2=CN=CC=C2
InChI : InChI=1S\/C10H12N2O\/c1-12-9(4-5-10(12)13)8-3-2-6-11-7-8\/h2-3,6-7,9H,4-5H2,1H3\/t9-\/m0\/s1
InChIKey : UIKROCXWUNQSPJ-VIFPVBQESA-N
Other name(s) : (-)-Cotinine,(S)-Cotinine,Cotinina
MW : 176.21
Formula : C10H12N2O
CAS_number : 486-56-6
PubChem : 854019
UniChem : UIKROCXWUNQSPJ-VIFPVBQESA-N
IUPHAR :
Wikipedia :
Target
Families : Cotinine ligand of proteins in family: Pectinacetylesterase-Notum
Stucture : 7BNF Human Notum comlexed with Cotinine
Protein : human-NOTUM
References (8)
| Title : Anxiolytic, Promnesic, Anti-Acetylcholinesterase and Antioxidant Effects of Cotinine and 6-Hydroxy-L-Nicotine in Scopolamine-Induced Zebrafish (Danio rerio) Model of Alzheimer's Disease - Boiangiu_2021_Antioxidants.(Basel)_10_ |
| Author(s) : Boiangiu RS , Mihasan M , Gorgan DL , Stache BA , Hritcu L |
| Ref : Antioxidants (Basel) , 10 : , 2021 |
| Abstract : Boiangiu_2021_Antioxidants.(Basel)_10_ |
| ESTHER : Boiangiu_2021_Antioxidants.(Basel)_10_ |
| PubMedSearch : Boiangiu_2021_Antioxidants.(Basel)_10_ |
| PubMedID: 33535660 |
| Gene_locus related to this paper: danre-ACHE |
| Title : Cotinine and 6-Hydroxy-L-Nicotine Reverses Memory Deficits and Reduces Oxidative Stress in Abeta(25-35)-Induced Rat Model of Alzheimer's Disease - Boiangiu_2020_Antioxidants.(Basel)_9_ |
| Author(s) : Boiangiu RS , Mihasan M , Gorgan DL , Stache BA , Petre BA , Hritcu L |
| Ref : Antioxidants (Basel) , 9 : , 2020 |
| Abstract : Boiangiu_2020_Antioxidants.(Basel)_9_ |
| ESTHER : Boiangiu_2020_Antioxidants.(Basel)_9_ |
| PubMedSearch : Boiangiu_2020_Antioxidants.(Basel)_9_ |
| PubMedID: 32824768 |
| Title : Evaluation of nicotine and cotinine analogs as potential neuroprotective agents for Alzheimer's disease - Gao_2014_Bioorg.Med.Chem.Lett_24_1472 |
| Author(s) : Gao J , Adam BL , Terry AV, Jr. |
| Ref : Bioorganic & Medicinal Chemistry Lett , 24 :1472 , 2014 |
| Abstract : Gao_2014_Bioorg.Med.Chem.Lett_24_1472 |
| ESTHER : Gao_2014_Bioorg.Med.Chem.Lett_24_1472 |
| PubMedSearch : Gao_2014_Bioorg.Med.Chem.Lett_24_1472 |
| PubMedID: 24581918 |
| Title : Poster: Acute administration of cotinine to DBA\/2 mice increases conditioning amplitude in the sensory inhibition model - |
| Author(s) : Stevens KE , Zheng L |
| Ref : Biochemical Pharmacology , 82 :1039 , 2011 |
| PubMedID: |
| Title : Cotinine reduces amyloid-beta aggregation and improves memory in Alzheimer's disease mice - Echeverria_2011_J.Alzheimers.Dis_24_817 |
| Author(s) : Echeverria V , Zeitlin R , Burgess S , Patel S , Barman A , Thakur G , Mamcarz M , Wang L , Sattelle DB , Kirschner DA , Mori T , Leblanc RM , Prabhakar R , Arendash GW |
| Ref : J Alzheimers Dis , 24 :817 , 2011 |
| Abstract : Echeverria_2011_J.Alzheimers.Dis_24_817 |
| ESTHER : Echeverria_2011_J.Alzheimers.Dis_24_817 |
| PubMedSearch : Echeverria_2011_J.Alzheimers.Dis_24_817 |
| PubMedID: 21321389 |
| Title : Poster: Chronic treatment with nicotine metabolite, cotinine, improves sustained attention and recognition memory in rats and attenuates glutamate (NMDA) antagonist-related impairments - |
| Author(s) : Terry AV, Jr. , Schade R , Callahan PM , Chapman JM , Bartlett MG |
| Ref : Biochemical Pharmacology , 82 :1041 , 2011 |
| PubMedID: |
| Title : (S)-(-)-Cotinine, the major brain metabolite of nicotine, stimulates nicotinic receptors to evoke [3H]dopamine release from rat striatal slices in a calcium-dependent manner - Dwoskin_1999_J.Pharmacol.Exp.Ther_288_905 |
| Author(s) : Dwoskin LP , Teng L , Buxton ST , Crooks PA |
| Ref : Journal of Pharmacology & Experimental Therapeutics , 288 :905 , 1999 |
| Abstract : Dwoskin_1999_J.Pharmacol.Exp.Ther_288_905 |
| ESTHER : Dwoskin_1999_J.Pharmacol.Exp.Ther_288_905 |
| PubMedSearch : Dwoskin_1999_J.Pharmacol.Exp.Ther_288_905 |
| PubMedID: 10027825 |
| Title : Pharmacokinetics of nicotine, cotinine, and 3'-hydroxycotinine in cigarette smokers - Scherer_1988_Klin.Wochenschr_66 Suppl 11_5 |
| Author(s) : Scherer G , Jarczyk L , Heller WD , Biber A , Neurath GB , Adlkofer F |
| Ref : Klin Wochenschr , 66 Suppl 11 :5 , 1988 |
| Abstract : Scherer_1988_Klin.Wochenschr_66 Suppl 11_5 |
| ESTHER : Scherer_1988_Klin.Wochenschr_66 Suppl 11_5 |
| PubMedSearch : Scherer_1988_Klin.Wochenschr_66 Suppl 11_5 |
| PubMedID: 3184779 |