Coelenteramide
Derives from an Oplophorus luciferin. The postcatalytic enzyme-product. || The most common marine luciferin. Luciferase from Renilla reniformis (RLuc) catalyzes the degradation of coelenterazine in the presence of molecular oxygen, resulting in the product coelenteramide, carbon dioxide, and the desired photon of light (EC 1.13.12.5). This enzyme belongs to the Haloalkane dehalogenase family II with a different catalytic function (EC 3.8.1.5) Reconstruction of an ancestral enzyme shows it has both hydrolase and monooxygenase activities ( Chaloupkova et al.)
General
Type : Natural,Luciferin
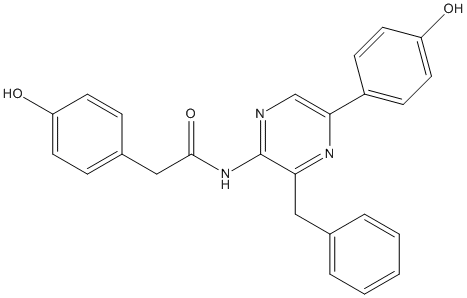
Chemical_Nomenclature : N-[3-benzyl-5-(4-hydroxyphenyl)pyrazin-2-yl]-2-(4-hydroxyphenyl)acetamide
Canonical SMILES : C1=CC=C(C=C1)CC2=NC(=CN=C2NC(=O)CC3=CC=C(C=C3)O)C4=CC=C(C=C4)O
InChI : InChI=1S\/C25H21N3O3\/c29-20-10-6-18(7-11-20)15-24(31)28-25-22(14-17-4-2-1-3-5-17)27-23(16-26-25)19-8-12-21(30)13-9-19\/h1-13,16,29-30H,14-15H2,(H,26,28,31)
InChIKey : CJIIERPDFZUYPI-UHFFFAOYSA-N
Other name(s) : DB04049,CEI,Oxidized Oplophorus luciferin,SCHEMBL134644,CHEBI:41487
MW : 411.5
Formula : C25H21N3O3
CAS_number : 50611-86-4
UniChem : CJIIERPDFZUYPI-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : Coelenteramide ligand of proteins in family: Haloalkane_dehalogenase-HLD2
Stucture :
Protein : renre-luc
References (4)
| Title : Catalytic mechanism for Renilla-type luciferases - Schenkmayerova_2023_Nat.Catal_6_23 |
| Author(s) : Schenkmayerova A , Toul M , Pluskal D , Baatallah R , Gagnot G , Pinto GP , Santana VT , Stuchla M , Neugebauer P , Chaiyen P , Damborsky J , Bednar D , Janin YL , Prokop Z , Marek M |
| Ref : Nature Catalysis , 6 :23 , 2023 |
| Abstract : Schenkmayerova_2023_Nat.Catal_6_23 |
| ESTHER : Schenkmayerova_2023_Nat.Catal_6_23 |
| PubMedSearch : Schenkmayerova_2023_Nat.Catal_6_23 |
| PubMedID: |
| Gene_locus related to this paper: 9zzzz-AncHLDRLuc2 , renre-luc |
| Title : Deciphering Enzyme Mechanisms with Engineered Ancestors and Substrate Analogues - Gao_2023_ChemCatChem_15_e202300745 |
| Author(s) : Gao T , Damborsky J , Janin YL , Marek M |
| Ref : ChemCatChem , 15 :e202300745 , 2023 |
| Abstract : Gao_2023_ChemCatChem_15_e202300745 |
| ESTHER : Gao_2023_ChemCatChem_15_e202300745 |
| PubMedSearch : Gao_2023_ChemCatChem_15_e202300745 |
| PubMedID: |
| Gene_locus related to this paper: renre-luc |
| Title : Engineering Protein Dynamics of Ancestral Luciferase - Schenkmayerova_2020_Chemrxiv__ |
| Author(s) : Schenkmayerova A , Pinto GP , Toul M , Marek M , Hernychova L , Planas-Iglesias J , Liskova V , Pluskal D , Vasina M , Emond S , Dorr M , Chaloupkova R , Bednar D , Prokop Z , Hollfelder F , Bornscheuer UT , Damborsky J |
| Ref : Chemrxiv , : , 2020 |
| Abstract : Schenkmayerova_2020_Chemrxiv__ |
| ESTHER : Schenkmayerova_2020_Chemrxiv__ |
| PubMedSearch : Schenkmayerova_2020_Chemrxiv__ |
| PubMedID: |
| Gene_locus related to this paper: renre-luc |
| Title : Crystal structures of the luciferase and green fluorescent protein from Renilla reniformis - Loening_2007_J.Mol.Biol_374_1017 |
| Author(s) : Loening AM , Fenn TD , Gambhir SS |
| Ref : Journal of Molecular Biology , 374 :1017 , 2007 |
| Abstract : Loening_2007_J.Mol.Biol_374_1017 |
| ESTHER : Loening_2007_J.Mol.Biol_374_1017 |
| PubMedSearch : Loening_2007_J.Mol.Biol_374_1017 |
| PubMedID: 17980388 |
| Gene_locus related to this paper: renre-luc |