Cisatracurium
Neuromuscular Nondepolarizing Agent, Nicotinic Antagonist, Neuromuscular blocking agent, (close to doxacurium) Cisatracurium is the 1R-cis 1'R-cis isomer of atracurium. Atracurium and Cisatracurium undergo spontaneous non-organ-dependent Hofmann degradation at physiological pH and temperature
General
Type : Propionate,Isoquinoline,Neuromuscular Nondepolarizing Agents,Nicotinic antagonist,Bisquaternary,Not A\/B H target
Chemical_Nomenclature : 5-[3-[(1R,2R)-1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-2-methyl-3,4-dihydro-1H-isoquinolin-2-ium-2-yl]propanoyloxy]pentyl
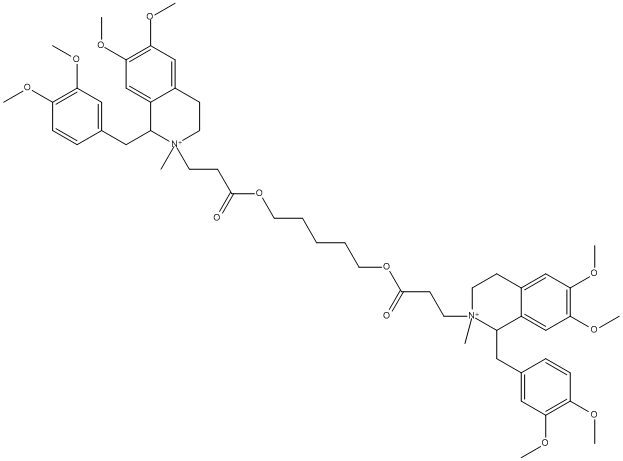
Canonical SMILES : C[N+]1(CCC2=CC(=C(C=C2C1CC3=CC(=C(C=C3)OC)OC)OC)OC)CCC(=O)OCCCCCOC(=O)CC[N+]4(CCC5=CC(=C(C=C5C4CC6=CC(=C(C=C6)OC)OC)OC)OC)C
InChI : InChI=1S\/C53H72N2O12\/c1-54(22-18-38-32-48(62-7)50(64-9)34-40(38)42(54)28-36-14-16-44(58-3)46(30-36)60-5)24-20-52(56)66-26-12-11-13-27-67-53(57)21-25-55(2)23-19-39-33-49(63-8)51(65-10)35-41(39)43(55)29-37-15-17-45(59-4)47(31-37)61-6\/h14-17,30-35,42-43H,11-13,18-29H2,1-10H3\/q+2\/t42-,43-,54-,55-\/m1\/s1
InChIKey : YXSLJKQTIDHPOT-LJCJQEJUSA-N
Other name(s) : cisatracurium besylate,3-[(1R,2R)-1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-2-methyl-3,4-dihydro-1H-isoquinolin-2-ium-2-yl]propanoate
MW : 929.14
Formula : C53H72N2O12
CAS_number : 96946-42-8
PubChem : 62887
UniChem : YXSLJKQTIDHPOT-LJCJQEJUSA-N
IUPHAR :
Wikipedia : Cisatracurium_besilate
Target
References (3)
| Title : Clinical pharmacokinetics of the newer neuromuscular blocking drugs - Atherton_1999_Clin.Pharmacokinet_36_169 |
| Author(s) : Atherton DP , Hunter JM |
| Ref : Clinical Pharmacokinetics , 36 :169 , 1999 |
| Abstract : Atherton_1999_Clin.Pharmacokinet_36_169 |
| ESTHER : Atherton_1999_Clin.Pharmacokinet_36_169 |
| PubMedSearch : Atherton_1999_Clin.Pharmacokinet_36_169 |
| PubMedID: 10223167 |
| Title : [The clinical pharmacology of mivacurium] - Diefenbach_1997_Anaesthesist_46_385 |
| Author(s) : Diefenbach C , Mellinghoff H |
| Ref : Anaesthesist , 46 :385 , 1997 |
| Abstract : Diefenbach_1997_Anaesthesist_46_385 |
| ESTHER : Diefenbach_1997_Anaesthesist_46_385 |
| PubMedSearch : Diefenbach_1997_Anaesthesist_46_385 |
| PubMedID: 9245207 |
| Title : [Esters and stereoisomers] - Nigrovic_1997_Anaesthesist_46_282 |
| Author(s) : Nigrovic V , Diefenbach C , Mellinghoff H |
| Ref : Anaesthesist , 46 :282 , 1997 |
| Abstract : Nigrovic_1997_Anaesthesist_46_282 |
| ESTHER : Nigrovic_1997_Anaesthesist_46_282 |
| PubMedSearch : Nigrovic_1997_Anaesthesist_46_282 |
| PubMedID: 9229981 |