Chlorpyrifos-methyl-oxon
General
Type : Insecticide,Organophosphate
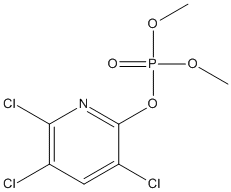
Chemical_Nomenclature : dimethyl (3,5,6-trichloropyridin-2-yl) phosphate
Canonical SMILES : COP(=O)(OC)OC1=NC(=C(C=C1Cl)Cl)Cl
InChI : InChI=1S\/C7H7Cl3NO4P\/c1-13-16(12,14-2)15-7-5(9)3-4(8)6(10)11-7\/h3H,1-2H3
InChIKey : XCBOKUAJQWDYNI-UHFFFAOYSA-N
Other name(s) : 3,5,6-trichloro-2-pyridyl dimethyl phosphate,2,3,5-trichloro-6-dimethoxyphosphoryloxy-pyridine,Fospirate,Torelle,Fospirate methyl,Caswell No. 465CC,Chlorpyrifos methyl oxon,Chlorpyrifos-methyl oxon
MW : 322.533
Formula : C7H7Cl3NO4P
CAS_number :
PubChem : 21805
UniChem : XCBOKUAJQWDYNI-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
References (3)
| Title : Inhibition properties of three acetylcholinesterases of the pinewood nematode Bursaphelenchus xylophilus by organophosphates and carbamates - Kang_2012_Pestic.Biochem.Physiol_104_157 |
| Author(s) : Kang JS , Moon YS , Lee SH |
| Ref : Pesticide Biochemistry and Physiology , 104 :157 , 2012 |
| Abstract : Kang_2012_Pestic.Biochem.Physiol_104_157 |
| ESTHER : Kang_2012_Pestic.Biochem.Physiol_104_157 |
| PubMedSearch : Kang_2012_Pestic.Biochem.Physiol_104_157 |
| PubMedID: |
| Gene_locus related to this paper: burxy-ACHE1 , burxy-ACHE2 , burxy-ACHE3 |
| Title : Evaluation of the Role of Boll Weevil Aliesterases in Noncatalytic Detoxication of Four Organophosphorus Insecticides - Yuan_1998_Pestic.Biochem.Physiol_61_135 |
| Author(s) : Yuan J , Chambers HW |
| Ref : Pesticide Biochemistry and Physiology , 61 :135 , 1998 |
| Abstract : Yuan_1998_Pestic.Biochem.Physiol_61_135 |
| ESTHER : Yuan_1998_Pestic.Biochem.Physiol_61_135 |
| PubMedSearch : Yuan_1998_Pestic.Biochem.Physiol_61_135 |
| PubMedID: |
| Title : Automated sample clean-up and fractionation of chlorpyrifos, chlorpyrifos-methyl and metabolites in mussels using normal-phase liquid chromatography - Serrano_1997_J.Chromatogr.A_778_151 |
| Author(s) : Serrano R , Lopez FJ , Roig-Navarro A , Hernandez F |
| Ref : Journal of Chromatography A , 778 :151 , 1997 |
| Abstract : Serrano_1997_J.Chromatogr.A_778_151 |
| ESTHER : Serrano_1997_J.Chromatogr.A_778_151 |
| PubMedSearch : Serrano_1997_J.Chromatogr.A_778_151 |
| PubMedID: 9299732 |