Chlorpromazine
The prototypical phenothiazine antipsychotic drug. Like the other drugs in this class chlorpromazine's antipsychotic actions are thought to be due to long-term adaptation by the brain to blocking dopamine receptors. It is one of the most sedating of the typical antipsychotics and also causes antimuscarinic effects. Tricyclic amine-containing compound
General
Type : Not A\/B H target,Phenothiazine,Multitarget,Drug,Sulfur Compound
Chemical_Nomenclature : 8-chlorpromazine, 2-chloro-N,N-dimethyl-10H-Phenothiazine-10-propanamine
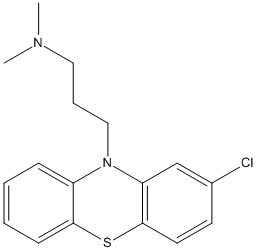
Canonical SMILES : CN(C)CCCN1C2=CC=CC=C2SC3=C1C=C(C=C3)Cl
InChI : InChI=1S\/C17H19ClN2S\/c1-19(2)10-5-11-20-14-6-3-4-7-16(14)21-17-9-8-13(18)12-15(17)20\/h3-4,6-9,12H,5,10-11H2,1-2H3
InChIKey : ZPEIMTDSQAKGNT-UHFFFAOYSA-N
Other name(s) : Propaphenin,Largactil,Thorazin,Contomin,Megaphen,Cromedazine,Plegomazin,Chloropromazine,Chlorderazin,Thorazine,Ormazine,Thor-prom
MW : 355.3-318.86
Formula : C17H19ClN2S
CAS_number : 69-09-0 || 50-53-3
PubChem : 2726
UniChem : ZPEIMTDSQAKGNT-UHFFFAOYSA-N
IUPHAR : 83
Wikipedia : Chlorpromazine
Target
References (6)
| Title : Inactivation of cholinesterase induced by chlorpromazine cation radicals - Muraoka_2003_Pharmacol.Toxicol_92_100 |
| Author(s) : Muraoka S , Miura T |
| Ref : Pharmacol Toxicol , 92 :100 , 2003 |
| Abstract : Muraoka_2003_Pharmacol.Toxicol_92_100 |
| ESTHER : Muraoka_2003_Pharmacol.Toxicol_92_100 |
| PubMedSearch : Muraoka_2003_Pharmacol.Toxicol_92_100 |
| PubMedID: 12747580 |
| Title : Inhibition of butyrylcholinesterase by phenothiazine derivatives - Debord_2002_J.Enzyme.Inhib.Med.Chem_17_197 |
| Author(s) : Debord J , Merle L , Bollinger JC , Dantoine T |
| Ref : J Enzyme Inhib Med Chem , 17 :197 , 2002 |
| Abstract : Debord_2002_J.Enzyme.Inhib.Med.Chem_17_197 |
| ESTHER : Debord_2002_J.Enzyme.Inhib.Med.Chem_17_197 |
| PubMedSearch : Debord_2002_J.Enzyme.Inhib.Med.Chem_17_197 |
| PubMedID: 12443046 |
| Title : Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase inhibitors - Radic_1993_Biochemistry_32_12074 |
| Author(s) : Radic Z , Pickering NA , Vellom DC , Camp S , Taylor P |
| Ref : Biochemistry , 32 :12074 , 1993 |
| Abstract : Radic_1993_Biochemistry_32_12074 |
| ESTHER : Radic_1993_Biochemistry_32_12074 |
| PubMedSearch : Radic_1993_Biochemistry_32_12074 |
| PubMedID: 8218285 |
| Title : Genetic variants of human serum cholinesterase influence metabolism of the muscle relaxant succinylcholine. - Lockridge_1990_Pharmacol.Ther_47_35 |
| Author(s) : Lockridge O |
| Ref : Pharmacol Ther , 47 :35 , 1990 |
| Abstract : Lockridge_1990_Pharmacol.Ther_47_35 |
| ESTHER : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedSearch : Lockridge_1990_Pharmacol.Ther_47_35 |
| PubMedID: 2195556 |
| Gene_locus related to this paper: human-BCHE |
| Title : Differential inhibition of plasma cholinesterase variants using the dibutyrate analogue of pancuronium bromide - Whittaker_1981_Hum.Hered_31_242 |
| Author(s) : Whittaker M , Britten JJ |
| Ref : Hum Hered , 31 :242 , 1981 |
| Abstract : Whittaker_1981_Hum.Hered_31_242 |
| ESTHER : Whittaker_1981_Hum.Hered_31_242 |
| PubMedSearch : Whittaker_1981_Hum.Hered_31_242 |
| PubMedID: 7287015 |