Carbachol
Carbachol is a synthetic choline ester and a positively charged quaternary ammonium compound. Carbachol is a parasympathomimetic that mimics the effect of acetylcholine on both the muscarinic and nicotinic receptors. This drug is administered ocularly to induce miosis to reduce intraocular pressure in the treatment of glaucoma. Carbachol is also used to stimulate micturition by contraction of detrusor muscle. This drug may cause hypotension, bradycardia, nausea, vomiting, bronchospasm, and abdominal cramps. As a carbamate it is a slowly hydrolyzed substrate of acetylcholinesterase
General
Type : Not A\/B H target,Carbamate,Trimethylammonium,Analogue of substrate,Drug
Chemical_Nomenclature : 2-carbamoyloxyethyl(trimethyl)azanium\;chloride
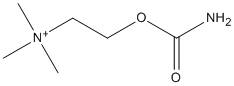
Canonical SMILES : C[N+](C)(C)CCOC(=O)N.[Cl-]
InChI : InChI=1S\/C6H14N2O2.ClH\/c1-8(2,3)4-5-10-6(7)9\;\/h4-5H2,1-3H3,(H-,7,9)\;1H
InChIKey : AIXAANGOTKPUOY-UHFFFAOYSA-N
Other name(s) : Carbamylcholine,Carbamoylcholine,Carbastat,Carbacholin,Carbacholine,Carbacolina,Carbamiotin,Carbocholin,Carbocholine,Carbochol,Carcholin,Miostat,Carbacholinum,Karbachol,2-[(Aminocarbonyl)oxy]-N,N,N-trimethylethanaminium chloride,(2-hydroxyethyl)trimethylammonium chloride carbamate,2-carbamoyloxyethyl-trimethyl-ammonium
MW : 147.196
Formula : C6H15N2O2
CAS_number : 51-83-2
UniChem : AIXAANGOTKPUOY-UHFFFAOYSA-N
IUPHAR :
Wikipedia : Carbachol
Target
References (3)
| Title : Molecular basis of inhibition of substrate hydrolysis by a ligand bound to the peripheral site of acetylcholinesterase - Auletta_2010_Chem.Biol.Interact_187_135 |
| Author(s) : Auletta JT , Johnson JL , Rosenberry TL |
| Ref : Chemico-Biological Interactions , 187 :135 , 2010 |
| Abstract : Auletta_2010_Chem.Biol.Interact_187_135 |
| ESTHER : Auletta_2010_Chem.Biol.Interact_187_135 |
| PubMedSearch : Auletta_2010_Chem.Biol.Interact_187_135 |
| PubMedID: 20493829 |
| Title : Analysis of the reaction of carbachol with acetylcholinesterase using thioflavin T as a coupled fluorescence reporter - Rosenberry_2008_Biochemistry_47_13056 |
| Author(s) : Rosenberry TL , Sonoda LK , Dekat SE , Cusack B , Johnson JL |
| Ref : Biochemistry , 47 :13056 , 2008 |
| Abstract : Rosenberry_2008_Biochemistry_47_13056 |
| ESTHER : Rosenberry_2008_Biochemistry_47_13056 |
| PubMedSearch : Rosenberry_2008_Biochemistry_47_13056 |
| PubMedID: 19006330 |
| Title : Monitoring the reaction of carbachol with acetylcholinesterase by thioflavin T fluorescence and acetylthiocholine hydrolysis - Rosenberry_2008_Chem.Biol.Interact_175_235 |
| Author(s) : Rosenberry TL , Sonoda LK , Dekat SE , Cusack B , Johnson JL |
| Ref : Chemico-Biological Interactions , 175 :235 , 2008 |
| Abstract : Rosenberry_2008_Chem.Biol.Interact_175_235 |
| ESTHER : Rosenberry_2008_Chem.Biol.Interact_175_235 |
| PubMedSearch : Rosenberry_2008_Chem.Biol.Interact_175_235 |
| PubMedID: 18602908 |