Caffeic-acid
Product of hydrolysis by Faec and inhibitor of some a/b hydrolases. Caffeic Acid is an orally bioavailable, hydroxycinnamic acid derivative and polyphenol, A member of catechols, with potential anti-oxidant, anti-inflammatory, and antineoplastic activities. It has a role as a plant metabolite, an EC 1.13.11.33 (arachidonate 15-lipoxygenase) inhibitor, an EC 2.5.1.18 (glutathione transferase) inhibitor, an EC 1.13.11.34 (arachidonate 5-lipoxygenase) inhibitor, an antioxidant and an EC 3.5.1.98 (histone deacetylase) inhibitor.
General
Type : Hydroxycinnamic-ester,Acrylate
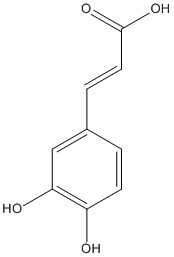
Chemical_Nomenclature : (E)-3-(3,4-dihydroxyphenyl)prop-2-enoic acid
Canonical SMILES : C1=CC(=C(C=C1C=CC(=O)O)O)O
InChI : InChI=1S\/C9H8O4\/c10-7-3-1-6(5-8(7)11)2-4-9(12)13\/h1-5,10-11H,(H,12,13)\/b4-2+
InChIKey : QAIPRVGONGVQAS-DUXPYHPUSA-N
Other name(s) : Caffeic acid,3,4-Dihydroxycinnamic acid,331-39-5,3-(3,4-dihydroxyphenyl)acrylic acid,Trans-Caffeate,DB01880,CHEBI:16433,CHEBI:36281,CHEMBL145
MW : 180.15
Formula : C9H8O4
CAS_number : 501-16-6
PubChem : 689043
UniChem : QAIPRVGONGVQAS-DUXPYHPUSA-N
IUPHAR :
Wikipedia :
Target
Families : Caffeic-acid ligand of proteins in family: FAE-Bacterial-promiscuous
Stucture : 3S2Z Crystal structure of the Lactobacillus johnsonii cinnamoyl esterase LJ0536 S106A mutant in complex with caffeic acid (replaced 3PFA: withdrawn)
Protein : lacjo-q74hk5
References (2)
| Title : Modulatory effect of caffeic acid on cholinesterases inhibitory properties of donepezil - Agunloye_2017_J.Complement.Integr.Med_15_ |
| Author(s) : Agunloye OM , Oboh G |
| Ref : J Complement Integr Med , 15 : , 2017 |
| Abstract : Agunloye_2017_J.Complement.Integr.Med_15_ |
| ESTHER : Agunloye_2017_J.Complement.Integr.Med_15_ |
| PubMedSearch : Agunloye_2017_J.Complement.Integr.Med_15_ |
| PubMedID: 28941354 |
| Title : An inserted alpha\/beta subdomain shapes the catalytic pocket of Lactobacillus johnsonii cinnamoyl esterase - Lai_2011_PLoS.One_6_e23269 |
| Author(s) : Lai KK , Stogios PJ , Vu C , Xu X , Cui H , Molloy S , Savchenko A , Yakunin A , Gonzalez CF |
| Ref : PLoS ONE , 6 :e23269 , 2011 |
| Abstract : Lai_2011_PLoS.One_6_e23269 |
| ESTHER : Lai_2011_PLoS.One_6_e23269 |
| PubMedSearch : Lai_2011_PLoS.One_6_e23269 |
| PubMedID: 21876742 |
| Gene_locus related to this paper: lacjo-q74hk5 |