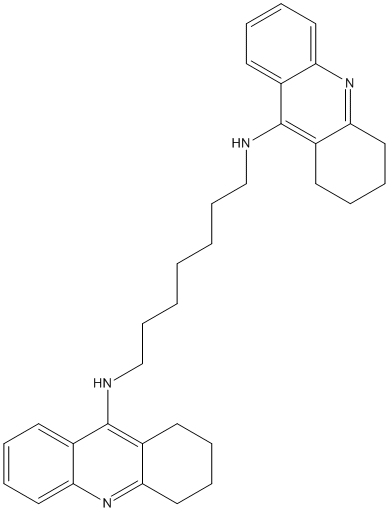
Bis7-tacrine
bis-ligand derived from used to increase AChE inhibition || rat brain AChE IC50 (nM) 1.5+/-0.3; rat serum BuChE IC50(nM) 149+/-23
General
Type : Derivative of Tacrine,Alkyl linked bis-ligand,Acridine
Chemical_Nomenclature : 1,7-N-Heptylene-bis-9,9'-amino-1,2,3,4-tetrahydroacridine
Canonical SMILES : C1CCC2=NC3=CC=CC=C3C(=C2C1)NCCCCCCCNC4=C5CCCCC5=NC6=CC=CC=C64
InChI : InChI=1S\/C33H40N4\/c1(2-12-22-34-32-24-14-4-8-18-28(24)36-29-19-9-5-15-25(29)32)3-13-23-35-33-26-16-6-10-20-30(26)37-31-21-11-7-17-27(31)33\/h4,6,8,10,14,16,18,20H,1-3,5,7,9,11-13,15,17,19,21-23H2,(H,34,36)(H,35,37)
InChIKey : ITZOKHKOFJOBFS-UHFFFAOYSA-N
Other name(s) : Heptylene-bis-tacrine,Heptylene-bis-(THA)dihydrochloride,Bis(7)-Tacrine,CHEMBL32823,Tacrine-Based Inhibitor 2f,Bis-7-Tacrine,bis(7)-cognitin,AA7
MW : 565.64
Formula : C33H40N4.2HCl
CAS_number :
UniChem : ITZOKHKOFJOBFS-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (28)
| Title : Promising tacrine\/huperzine A-based dimeric acetylcholinesterase inhibitors for neurodegenerative disorders: From relieving symptoms to modifying diseases through multitarget - Mak_2021_J.Neurochem__ |
| Author(s) : Mak S , Li W , Fu H , Luo J , Cui W , Hu S , Pang Y , Carlier PR , Tsim KWK , Pi R , Han Y |
| Ref : Journal of Neurochemistry , : , 2021 |
| Abstract : Mak_2021_J.Neurochem__ |
| ESTHER : Mak_2021_J.Neurochem__ |
| PubMedSearch : Mak_2021_J.Neurochem__ |
| PubMedID: 33930191 |
| Title : Potent Acetylcholinesterase Selective and Reversible Homodimeric Agent Based on Tacrine for Theranostics - Meena_2019_Mol.Pharm_16_2296 |
| Author(s) : Meena VK , Chaturvedi S , Sharma RK , Mishra AK , Hazari PP |
| Ref : Mol Pharm , 16 :2296 , 2019 |
| Abstract : Meena_2019_Mol.Pharm_16_2296 |
| ESTHER : Meena_2019_Mol.Pharm_16_2296 |
| PubMedSearch : Meena_2019_Mol.Pharm_16_2296 |
| PubMedID: 31059278 |
| Title : Pro-cognitive effect of bis(7)-tacrine as potential therapeutic agent against neurodegenerative disorders - Korabecny_2018_Mil.Med.Sci.Lett_87_34 |
| Author(s) : Korabecny J |
| Ref : Military Medical Science Letters , 87 :34 , 2018 |
| Abstract : Korabecny_2018_Mil.Med.Sci.Lett_87_34 |
| ESTHER : Korabecny_2018_Mil.Med.Sci.Lett_87_34 |
| PubMedSearch : Korabecny_2018_Mil.Med.Sci.Lett_87_34 |
| PubMedID: |
| Title : On the use of the experimentally determined enzyme inhibition constant as a measure of absolute binding affinity - Darras_2017_Biochem.Biophys.Res.Commun_489_451 |
| Author(s) : Darras FH , Pang YP |
| Ref : Biochemical & Biophysical Research Communications , 489 :451 , 2017 |
| Abstract : Darras_2017_Biochem.Biophys.Res.Commun_489_451 |
| ESTHER : Darras_2017_Biochem.Biophys.Res.Commun_489_451 |
| PubMedSearch : Darras_2017_Biochem.Biophys.Res.Commun_489_451 |
| PubMedID: 28571743 |
| Title : Synthesis, biological activity, and biopharmaceutical characterization of tacrine dimers as acetylcholinesterase inhibitors - Qian_2014_Int.J.Pharm_477_442 |
| Author(s) : Qian S , He L , Mak M , Han Y , Ho CY , Zuo Z |
| Ref : Int J Pharm , 477 :442 , 2014 |
| Abstract : Qian_2014_Int.J.Pharm_477_442 |
| ESTHER : Qian_2014_Int.J.Pharm_477_442 |
| PubMedSearch : Qian_2014_Int.J.Pharm_477_442 |
| PubMedID: 25445524 |
| Title : Inhibition of human carboxylesterases hCE1 and hiCE by cholinesterase inhibitors - Tsurkan_2013_Chem.Biol.Interact_203_226 |
| Author(s) : Tsurkan LG , Hatfield MJ , Edwards CC , Hyatt JL , Potter PM |
| Ref : Chemico-Biological Interactions , 203 :226 , 2013 |
| Abstract : Tsurkan_2013_Chem.Biol.Interact_203_226 |
| ESTHER : Tsurkan_2013_Chem.Biol.Interact_203_226 |
| PubMedSearch : Tsurkan_2013_Chem.Biol.Interact_203_226 |
| PubMedID: 23123248 |
| Title : Effect of bis(7)-tacrine on cognition in rats with chronic cerebral ischemia - Shu_2012_Neurosci.Lett_512_103 |
| Author(s) : Shu XJ , Liu W , Zhang L , Yang R , Yi HL , Li CL , Ye YJ , Ai YX |
| Ref : Neuroscience Letters , 512 :103 , 2012 |
| Abstract : Shu_2012_Neurosci.Lett_512_103 |
| ESTHER : Shu_2012_Neurosci.Lett_512_103 |
| PubMedSearch : Shu_2012_Neurosci.Lett_512_103 |
| PubMedID: 22330749 |
| Title : Multi-target strategy to address Alzheimer's disease: design, synthesis and biological evaluation of new tacrine-based dimers - Rizzo_2011_Eur.J.Med.Chem_46_4336 |
| Author(s) : Rizzo S , Bisi A , Bartolini M , Mancini F , Belluti F , Gobbi S , Andrisano V , Rampa A |
| Ref : Eur Journal of Medicinal Chemistry , 46 :4336 , 2011 |
| Abstract : Rizzo_2011_Eur.J.Med.Chem_46_4336 |
| ESTHER : Rizzo_2011_Eur.J.Med.Chem_46_4336 |
| PubMedSearch : Rizzo_2011_Eur.J.Med.Chem_46_4336 |
| PubMedID: 21798635 |
| Title : Mechanism of bis(7)-tacrine inhibition of GABA-activated current in cultured rat hippocampal neurons - Zhou_2009_Neuropharmacol_57_33 |
| Author(s) : Zhou L , Liu YW , Peoples RW , Yang M , Tian X , Ai YX , Pang YP , Li ZW , Han YF , Li CY |
| Ref : Neuropharmacology , 57 :33 , 2009 |
| Abstract : Zhou_2009_Neuropharmacol_57_33 |
| ESTHER : Zhou_2009_Neuropharmacol_57_33 |
| PubMedSearch : Zhou_2009_Neuropharmacol_57_33 |
| PubMedID: 19393253 |
| Title : Comparison studies of tacrine and bis7-tacrine on the suppression of scopolamine-induced behavioral changes and inhibition of acetylcholinesterase in mice - Pan_2009_Pharmacology_83_294 |
| Author(s) : Pan SY , Yu ZL , Xiang CJ , Dong H , Fang HY , Ko KM |
| Ref : Pharmacology , 83 :294 , 2009 |
| Abstract : Pan_2009_Pharmacology_83_294 |
| ESTHER : Pan_2009_Pharmacology_83_294 |
| PubMedSearch : Pan_2009_Pharmacology_83_294 |
| PubMedID: 19365154 |
| Title : Inhibition of NMDA-gated ion channels by bis(7)-tacrine: whole-cell and single-channel studies - Liu_2008_Neuropharmacol_54_1086 |
| Author(s) : Liu YW , Luo JL , Ren H , Peoples RW , Ai YX , Liu LJ , Pang YP , Li ZW , Han YF , Li CY |
| Ref : Neuropharmacology , 54 :1086 , 2008 |
| Abstract : Liu_2008_Neuropharmacol_54_1086 |
| ESTHER : Liu_2008_Neuropharmacol_54_1086 |
| PubMedSearch : Liu_2008_Neuropharmacol_54_1086 |
| PubMedID: 18407299 |
| Title : The physicochemical properties and the in vivo AChE inhibition of two potential anti-Alzheimer agents, bis(12)-hupyridone and bis(7)-tacrine - Yu_2008_J.Pharm.Biomed.Anal_46_75 |
| Author(s) : Yu H , Li WM , Kan KK , Ho JM , Carlier PR , Pang YP , Gu ZM , Zhong Z , Chan K , Wang YT , Han YF |
| Ref : J Pharm Biomed Anal , 46 :75 , 2008 |
| Abstract : Yu_2008_J.Pharm.Biomed.Anal_46_75 |
| ESTHER : Yu_2008_J.Pharm.Biomed.Anal_46_75 |
| PubMedSearch : Yu_2008_J.Pharm.Biomed.Anal_46_75 |
| PubMedID: 17931815 |
| Title : Selective and sensitive determination of bis(7)-tacrine, a high erythrocyte binding acetylcholinesterase inhibitor, in rat plasma by high-performance liquid chromatography-tandem mass spectrometry - Zhang_2008_Biomed.Chromatogr_22_414 |
| Author(s) : Zhang L , Yu H , Li WM , Cheung MC , Pang YP , Lin G , Wang YT , Zuo Z , Han YF |
| Ref : Biomedical Chromatography , 22 :414 , 2008 |
| Abstract : Zhang_2008_Biomed.Chromatogr_22_414 |
| ESTHER : Zhang_2008_Biomed.Chromatogr_22_414 |
| PubMedSearch : Zhang_2008_Biomed.Chromatogr_22_414 |
| PubMedID: 18059054 |
| Title : Multi-target-directed drug design strategy: from a dual binding site acetylcholinesterase inhibitor to a trifunctional compound against Alzheimer's disease - Bolognesi_2007_J.Med.Chem_50_6446 |
| Author(s) : Bolognesi ML , Cavalli A , Valgimigli L , Bartolini M , Rosini M , Andrisano V , Recanatini M , Melchiorre C |
| Ref : Journal of Medicinal Chemistry , 50 :6446 , 2007 |
| Abstract : Bolognesi_2007_J.Med.Chem_50_6446 |
| ESTHER : Bolognesi_2007_J.Med.Chem_50_6446 |
| PubMedSearch : Bolognesi_2007_J.Med.Chem_50_6446 |
| PubMedID: 18047264 |
| Title : East meets West in the search for Alzheimer's therapeutics - novel dimeric inhibitors from tacrine and huperzine A - Li_2007_Curr.Alzheimer.Res_4_386 |
| Author(s) : Li WM , Kan KK , Carlier PR , Pang YP , Han YF |
| Ref : Curr Alzheimer Res , 4 :386 , 2007 |
| Abstract : Li_2007_Curr.Alzheimer.Res_4_386 |
| ESTHER : Li_2007_Curr.Alzheimer.Res_4_386 |
| PubMedSearch : Li_2007_Curr.Alzheimer.Res_4_386 |
| PubMedID: 17908041 |
| Title : Mitochondrial proteomic analysis and characterization of the intracellular mechanisms of bis(7)-tacrine in protecting against glutamate-induced excitotoxicity in primary cultured neurons - Fu_2007_J.Proteome.Res_6_2435 |
| Author(s) : Fu H , Li W , Liu Y , Lao Y , Liu W , Chen C , Yu H , Lee NT , Chang DC , Li P , Pang Y , Tsim KWK , Li M , Han Y |
| Ref : J Proteome Res , 6 :2435 , 2007 |
| Abstract : Fu_2007_J.Proteome.Res_6_2435 |
| ESTHER : Fu_2007_J.Proteome.Res_6_2435 |
| PubMedSearch : Fu_2007_J.Proteome.Res_6_2435 |
| PubMedID: 17530875 |
| Title : Neuroprotection via inhibition of nitric oxide synthase by bis(7)-tacrine - Li_2006_Neuroreport_17_471 |
| Author(s) : Li W , Lee NT , Fu H , Kan KK , Pang Y , Li M , Tsim KWK , Li X , Han Y |
| Ref : Neuroreport , 17 :471 , 2006 |
| Abstract : Li_2006_Neuroreport_17_471 |
| ESTHER : Li_2006_Neuroreport_17_471 |
| PubMedSearch : Li_2006_Neuroreport_17_471 |
| PubMedID: 16543809 |
| Title : Complexes of alkylene-linked tacrine dimers with Torpedo californica acetylcholinesterase: Binding of Bis5-tacrine produces a dramatic rearrangement in the active-site gorge - Rydberg_2006_J.Med.Chem_49_5491 |
| Author(s) : Rydberg EH , Brumshtein B , Greenblatt HM , Wong DM , Shaya D , Williams LD , Carlier PR , Pang YP , Silman I , Sussman JL |
| Ref : Journal of Medicinal Chemistry , 49 :5491 , 2006 |
| Abstract : Rydberg_2006_J.Med.Chem_49_5491 |
| ESTHER : Rydberg_2006_J.Med.Chem_49_5491 |
| PubMedSearch : Rydberg_2006_J.Med.Chem_49_5491 |
| PubMedID: 16942022 |
| Gene_locus related to this paper: torca-ACHE |
| Title : Bis(7)-tacrine attenuates beta amyloid-induced neuronal apoptosis by regulating L-type calcium channels - Fu_2006_J.Neurochem_98_1400 |
| Author(s) : Fu H , Li W , Lao Y , Luo J , Lee NT , Kan KK , Tsang HW , Tsim KWK , Pang Y , Li Z , Chang DC , Li M , Han Y |
| Ref : Journal of Neurochemistry , 98 :1400 , 2006 |
| Abstract : Fu_2006_J.Neurochem_98_1400 |
| ESTHER : Fu_2006_J.Neurochem_98_1400 |
| PubMedSearch : Fu_2006_J.Neurochem_98_1400 |
| PubMedID: 16771827 |
| Title : Effects of bis(7)-tacrine on spontaneous synaptic activity and on the nicotinic ACh receptor of Torpedo electric organ - Ros_2001_J.Neurophysiol_86_183 |
| Author(s) : Ros E , Aleu J , Gomez de Aranda I , Canti C , Pang YP , Marsal J , Solsona C |
| Ref : Journal of Neurophysiology , 86 :183 , 2001 |
| Abstract : Ros_2001_J.Neurophysiol_86_183 |
| ESTHER : Ros_2001_J.Neurophysiol_86_183 |
| PubMedSearch : Ros_2001_J.Neurophysiol_86_183 |
| PubMedID: 11431500 |
| Title : Corrigendum to 'Protection against ischemic injury in primary cultured mouse astrocytes by bis(7)-tacrine, a novel acetylcholinesterase inhibitor' - |
| Author(s) : Wu D , Xiao X , Ng AK , Chen PM , Chung W , Lee NT , Carlier PR , Pang Y , Yu AC , Han Y |
| Ref : Neuroscience Letters , 290 :84 , 2000 |
| PubMedID: 10925180 |
| Title : Bis(7)-tacrine, a promising anti-Alzheimer's agent, reduces hydrogen peroxide-induced injury in rat pheochromocytoma cells: comparison with tacrine - Xiao_2000_Neurosci.Lett_290_197 |
| Author(s) : Xiao XQ , Lee NT , Carlier PR , Pang Y , Han YF |
| Ref : Neuroscience Letters , 290 :197 , 2000 |
| Abstract : Xiao_2000_Neurosci.Lett_290_197 |
| ESTHER : Xiao_2000_Neurosci.Lett_290_197 |
| PubMedSearch : Xiao_2000_Neurosci.Lett_290_197 |
| PubMedID: 10963897 |
| Title : Bis(7)-tacrine, a novel acetylcholinesterase inhibitor, reverses AF64A-induced deficits in navigational memory in rats - Liu_2000_Neurosci.Lett_282_165 |
| Author(s) : Liu J , Ho W , Lee NT , Carlier PR , Pang Y , Han Y |
| Ref : Neuroscience Letters , 282 :165 , 2000 |
| Abstract : Liu_2000_Neurosci.Lett_282_165 |
| ESTHER : Liu_2000_Neurosci.Lett_282_165 |
| PubMedSearch : Liu_2000_Neurosci.Lett_282_165 |
| PubMedID: 10717417 |
| Title : Protection against ischemic injury in primary cultured mouse astrocytes by bis(7)-tacrine, a novel acetylcholinesterase inhibitor [corrected] - Wu_2000_Neurosci.Lett_288_95 |
| Author(s) : Wu DC , Xiao XQ , Ng AK , Chen PM , Chung W , Lee NT , Carlier PR , Pang YP , Yu AC , Han YF |
| Ref : Neuroscience Letters , 288 :95 , 2000 |
| Abstract : Wu_2000_Neurosci.Lett_288_95 |
| ESTHER : Wu_2000_Neurosci.Lett_288_95 |
| PubMedSearch : Wu_2000_Neurosci.Lett_288_95 |
| PubMedID: 10876069 |
| Title : Bis(7)-tacrine, a novel dimeric AChE inhibitor, is a potent GABA(A) receptor antagonist - Li_1999_Neuroreport_10_795 |
| Author(s) : Li CY , Wang H , Xue H , Carlier PR , Hui KM , Pang YP , Li ZW , Han YF |
| Ref : Neuroreport , 10 :795 , 1999 |
| Abstract : Li_1999_Neuroreport_10_795 |
| ESTHER : Li_1999_Neuroreport_10_795 |
| PubMedSearch : Li_1999_Neuroreport_10_795 |
| PubMedID: 10208550 |
| Title : Effects of bis(7)-tacrine, a novel anti-Alzheimer's agent, on rat brain AChE - Wang_1999_Neuroreport_10_789 |
| Author(s) : Wang H , Carlier PR , Ho WL , Wu DC , Lee NT , Li CP , Pang YP , Han YF |
| Ref : Neuroreport , 10 :789 , 1999 |
| Abstract : Wang_1999_Neuroreport_10_789 |
| ESTHER : Wang_1999_Neuroreport_10_789 |
| PubMedSearch : Wang_1999_Neuroreport_10_789 |
| PubMedID: 10208549 |
| Title : Attenuation of scopolamine-induced deficits in navigational memory performance in rats by bis(7)-tacrine, a novel dimeric AChE inhibitor - Wang_1999_Zhongguo.Yao.Li.Xue.Bao_20_211 |
| Author(s) : Wang H , Carlier PR , Ho WL , Lee NT , Pang YP , Han YF |
| Ref : Zhongguo Yao Li Xue Bao , 20 :211 , 1999 |
| Abstract : Wang_1999_Zhongguo.Yao.Li.Xue.Bao_20_211 |
| ESTHER : Wang_1999_Zhongguo.Yao.Li.Xue.Bao_20_211 |
| PubMedSearch : Wang_1999_Zhongguo.Yao.Li.Xue.Bao_20_211 |
| PubMedID: 10452094 |
| Title : Highly potent, selective, and low cost bis-tetrahydroaminacrine inhibitors of acetylcholinesterase. Steps toward novel drugs for treating Alzheimer's disease - Pang_1996_J.Biol.Chem_271_23646 |
| Author(s) : Pang YP , Quiram P , Jelacic T , Hong F , Brimijoin S |
| Ref : Journal of Biological Chemistry , 271 :23646 , 1996 |
| Abstract : Pang_1996_J.Biol.Chem_271_23646 |
| ESTHER : Pang_1996_J.Biol.Chem_271_23646 |
| PubMedSearch : Pang_1996_J.Biol.Chem_271_23646 |
| PubMedID: 8798583 |