Benzenesulfonyl-fluoride
General
Type : Sulfur Compound,Sulfonyl
Chemical_Nomenclature : Benzenesulfonyl fluoride
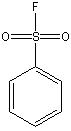
Canonical SMILES : C1=CC=C(C=C1)S(=O)(=O)F
InChI : InChI=1S\/C6H5FO2S\/c7-10(8,9)6-4-2-1-3-5-6\/h1-5H
InChIKey : IDIPWEYIBKUDNY-UHFFFAOYSA-N
Other name(s) : BSF,Phenylsulfonyl fluoride,Benzenesulphonyl fluoride,Phenylsulphonyl fluoride
MW : 160.2
Formula : C6H5FO2S
CAS_number : 368-43-4
PubChem : 67779
UniChem : IDIPWEYIBKUDNY-UHFFFAOYSA-N
Target
Families : Benzenesulfonyl-fluoride ligand of proteins in family
ACHE
References (3)
| Title : Inactivation studies of acetylcholinesterase with phenylmethylsulfonyl fluoride - Kraut_2000_Mol.Pharmacol_57_1243 |
| Author(s) : Kraut D , Goff H , Pai RK , Hosea NA , Silman I , Sussman JL , Taylor P , Voet JG |
| Ref : Molecular Pharmacology , 57 :1243 , 2000 |
| Abstract : Kraut_2000_Mol.Pharmacol_57_1243 |
| ESTHER : Kraut_2000_Mol.Pharmacol_57_1243 |
| PubMedSearch : Kraut_2000_Mol.Pharmacol_57_1243 |
| PubMedID: 10825396 |
| Title : [Partial characteristics of the basic phenylmethylsulfonylfluoride-inhibited carboxypeptidase from cat brain] - Vernigora_1995_Biokhimiia_60_1860 |
| Author(s) : Vernigora AN , Nikishin NN , Gengin MT |
| Ref : Biokhimiia , 60 :1860 , 1995 |
| Abstract : Vernigora_1995_Biokhimiia_60_1860 |
| ESTHER : Vernigora_1995_Biokhimiia_60_1860 |
| PubMedSearch : Vernigora_1995_Biokhimiia_60_1860 |
| PubMedID: 8590758 |
| Title : Sciatic nerve neuropathy target esterase. Methods of assay, proximo-distal distribution and regeneration - Barril_1988_Toxicology_49_107 |
| Author(s) : Barril JB , Vilanova E , Pellin MC |
| Ref : Toxicology , 49 :107 , 1988 |
| Abstract : Barril_1988_Toxicology_49_107 |
| ESTHER : Barril_1988_Toxicology_49_107 |
| PubMedSearch : Barril_1988_Toxicology_49_107 |
| PubMedID: 3376120 |