BW284C51
Inhibitor specific of AChE and used to differentiate AChE activity from BChE activity (inhibited by Iso-OMPA (Austin and Berry 1953) Before this publication distinction of AChE and BChE was difficult: Nu-1250, inhibitor of AChE and Ro-2-0683 or Nu-683 or diisopropylphosphorofluoridate (DFP) ( Hawkins & Mendel,1947) to which BChE appears to be about 100-200 times as sensitive as AChE, were used. Entry of reference human-ACHE
 The acetylcholinesterase inhibitor BW284c51 is a potent blocker of Torpedo nicotinic AchRs Olivera et al 2005
The acetylcholinesterase inhibitor BW284c51 is a potent blocker of Torpedo nicotinic AchRs Olivera et al 2005
General
Type : Bisquaternary
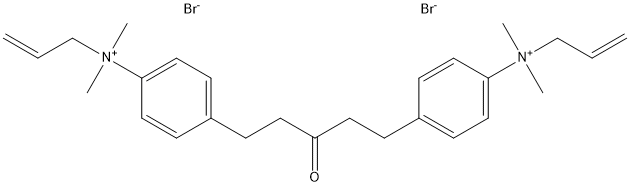
Chemical_Nomenclature : 1:5-bis(4-allyldimethylammoniumphenyl)-pentan-3-one dibromide || [4-[5-[4-[dimethyl(prop-2-enyl)azaniumyl]phenyl]-3-oxopentyl]phenyl]-dimethyl-prop-2-enylazanium
Canonical SMILES : C[N+](C)(CC=C)C1=CC=C(C=C1)CCC(=O)CCC2=CC=C(C=C2)[N+](C)(C)CC=C.[Br-].[Br-] || C[N+](C)(CC=C)C1=CC=C(C=C1)CCC(=O)CCC2=CC=C(C=C2)[N+](C)(C)CC=C
InChI : InChI=1S\/C27H38N2O.2BrH\/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2\;\;\/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3\;2*1H\/q+2\;\;\/p-2 || InChI=1S\/C27H38N2O\/c1-7-21-28(3,4)25-15-9-23(10-16-25)13-19-27(30)20-14-24-11-17-26(18-12-24)29(5,6)22-8-2\/h7-12,15-18H,1-2,13-14,19-22H2,3-6H3\/q+2
InChIKey : WKDURMTZOWGWTD-UHFFFAOYSA-L || ZAEXMNKDGJNLTA-UHFFFAOYSA-N
Other name(s) : BW297C50 is the corresponding diiodide,EBW,CHEMBL140020,BW-28C51,SCHEMBL7449688,ZINC3814201,BW 284 C 51,BW-284-C-51
MW : 566.4
Formula : C27H38N2OBr2
CAS_number : 402-40-4
UniChem : WKDURMTZOWGWTD-UHFFFAOYSA-L || ZAEXMNKDGJNLTA-UHFFFAOYSA-N
IUPHAR : 6600
Wikipedia :
Target
Families : BW284C51 ligand of proteins in family: ACHE
Stucture :
Protein : torca-ACHE || human-ACHE
References (27)
| Title : The acetylcholinesterase inhibitor BW284c51 is a potent blocker of Torpedo nicotinic AchRs incorporated into the Xenopus oocyte membrane - Olivera-Bravo_2005_Br.J.Pharmacol_144_88 |
| Author(s) : Olivera-Bravo S , Ivorra I , Morales A |
| Ref : British Journal of Pharmacology , 144 :88 , 2005 |
| Abstract : Olivera-Bravo_2005_Br.J.Pharmacol_144_88 |
| ESTHER : Olivera-Bravo_2005_Br.J.Pharmacol_144_88 |
| PubMedSearch : Olivera-Bravo_2005_Br.J.Pharmacol_144_88 |
| PubMedID: 15644872 |
| Title : Structure of a complex of the potent and specific inhibitor BW284C51 with Torpedo californica acetylcholinesterase - Felder_2002_Acta.Crystallogr.D.Biol.Crystallogr_58_1765 |
| Author(s) : Felder CE , Harel M , Silman I , Sussman JL |
| Ref : Acta Crystallographica D Biol Crystallogr , 58 :1765 , 2002 |
| Abstract : Felder_2002_Acta.Crystallogr.D.Biol.Crystallogr_58_1765 |
| ESTHER : Felder_2002_Acta.Crystallogr.D.Biol.Crystallogr_58_1765 |
| PubMedSearch : Felder_2002_Acta.Crystallogr.D.Biol.Crystallogr_58_1765 |
| PubMedID: 12351819 |
| Gene_locus related to this paper: torca-ACHE |
| Title : Inhibition of cholinesterase-associated aryl acylamidase activity by anticholinesterase agents: focus on drugs potentially effective in Alzheimer's disease - Costagli_1998_Biochem.Pharmacol_55_1733 |
| Author(s) : Costagli C , Galli A |
| Ref : Biochemical Pharmacology , 55 :1733 , 1998 |
| Abstract : Costagli_1998_Biochem.Pharmacol_55_1733 |
| ESTHER : Costagli_1998_Biochem.Pharmacol_55_1733 |
| PubMedSearch : Costagli_1998_Biochem.Pharmacol_55_1733 |
| PubMedID: 9634011 |
| Title : Acetylcholinesterase promotes regeneration of neurites in cultured adult neurons of Aplysia - Srivatsan_1997_Neurosci_77_921 |
| Author(s) : Srivatsan M , Peretz B |
| Ref : Neuroscience , 77 :921 , 1997 |
| Abstract : Srivatsan_1997_Neurosci_77_921 |
| ESTHER : Srivatsan_1997_Neurosci_77_921 |
| PubMedSearch : Srivatsan_1997_Neurosci_77_921 |
| PubMedID: 9070763 |
| Title : Purification and properties of monomeric (G1) forms of acetylcholinesterase secreted by Nippostrongylus brasiliensis - Grigg_1997_Mol.Biochem.Parasitol_90_513 |
| Author(s) : Grigg ME , Tang L , Hussein AS , Selkirk ME |
| Ref : Molecular & Biochemical Parasitology , 90 :513 , 1997 |
| Abstract : Grigg_1997_Mol.Biochem.Parasitol_90_513 |
| ESTHER : Grigg_1997_Mol.Biochem.Parasitol_90_513 |
| PubMedSearch : Grigg_1997_Mol.Biochem.Parasitol_90_513 |
| PubMedID: 98135665 |
| Title : Overlapping drug interaction sites of human butyrylcholinesterase dissected by site-directed mutagenesis - Loewenstein-Lichtenstein_1996_Mol.Pharmacol_50_1423 |
| Author(s) : Loewenstein-Lichtenstein Y , Glick D , Gluzman N , Sternfeld M , Zakut H , Soreq H |
| Ref : Molecular Pharmacology , 50 :1423 , 1996 |
| Abstract : Loewenstein-Lichtenstein_1996_Mol.Pharmacol_50_1423 |
| ESTHER : Loewenstein-Lichtenstein_1996_Mol.Pharmacol_50_1423 |
| PubMedSearch : Loewenstein-Lichtenstein_1996_Mol.Pharmacol_50_1423 |
| PubMedID: 8967962 |
| Gene_locus related to this paper: human-BCHE |
| Title : Cloning and expression of acetylcholinesterase from Bungarus fasciatus venom. A new type of cooh-terminal domain\; involvement of a positively charged residue in the peripheral site - Cousin_1996_J.Biol.Chem_271_15099 |
| Author(s) : Cousin X , Bon S , Duval N , Massoulie J , Bon C |
| Ref : Journal of Biological Chemistry , 271 :15099 , 1996 |
| Abstract : Cousin_1996_J.Biol.Chem_271_15099 |
| ESTHER : Cousin_1996_J.Biol.Chem_271_15099 |
| PubMedSearch : Cousin_1996_J.Biol.Chem_271_15099 |
| PubMedID: 8662867 |
| Gene_locus related to this paper: bunfa-ACHE |
| Title : Acetylcholinesterase in Dendrobaena veneta (Oligochaeta: Opisthopora) is present with forms sensitive and insensitive to phosphatidylinositol phospholipase C. Biochemical characterization and histochemical localization in the nervous system - Talesa_1996_Eur.J.Biochem_238_538 |
| Author(s) : Talesa V , Romani R , Rosi G , Giovannini E |
| Ref : European Journal of Biochemistry , 238 :538 , 1996 |
| Abstract : Talesa_1996_Eur.J.Biochem_238_538 |
| ESTHER : Talesa_1996_Eur.J.Biochem_238_538 |
| PubMedSearch : Talesa_1996_Eur.J.Biochem_238_538 |
| PubMedID: 8681969 |
| Title : The effect of acetylcholinesterase on outgrowth of dopaminergic neurons in organotypic slice culture of rat mid-brain - Jones_1995_Cell.Tiss.Res_279_323 |
| Author(s) : Jones SA , Holmes C , Budd TC , Greenfield SA |
| Ref : Cell & Tissue Research , 279 :323 , 1995 |
| Abstract : Jones_1995_Cell.Tiss.Res_279_323 |
| ESTHER : Jones_1995_Cell.Tiss.Res_279_323 |
| PubMedSearch : Jones_1995_Cell.Tiss.Res_279_323 |
| PubMedID: 7895271 |
| Title : Characterization of monoclonal antibodies that strongly inhibit Electrophorus electricus acetylcholinesterase - Remy_1995_Eur.J.Biochem_231_651 |
| Author(s) : Remy MH , Frobert Y , Grassi J |
| Ref : European Journal of Biochemistry , 231 :651 , 1995 |
| Abstract : Remy_1995_Eur.J.Biochem_231_651 |
| ESTHER : Remy_1995_Eur.J.Biochem_231_651 |
| PubMedSearch : Remy_1995_Eur.J.Biochem_231_651 |
| PubMedID: 7649165 |
| Title : Schistosoma: rate of glucose import is altered by acetylcholine interaction with tegumental acetylcholine receptors and acetylcholinesterase - Camacho_1995_Exp.Parasitol_81_584 |
| Author(s) : Camacho M , Agnew A |
| Ref : Experimental Parasitology , 81 :584 , 1995 |
| Abstract : Camacho_1995_Exp.Parasitol_81_584 |
| ESTHER : Camacho_1995_Exp.Parasitol_81_584 |
| PubMedSearch : Camacho_1995_Exp.Parasitol_81_584 |
| PubMedID: 8543000 |
| Title : Differential effects of peripheral site ligands on Torpedo and chicken acetylcholinesterase - Eichler_1994_Mol.Pharmacol_45_335 |
| Author(s) : Eichler J , Anselmet A , Sussman JL , Massoulie J , Silman I |
| Ref : Molecular Pharmacology , 45 :335 , 1994 |
| Abstract : Eichler_1994_Mol.Pharmacol_45_335 |
| ESTHER : Eichler_1994_Mol.Pharmacol_45_335 |
| PubMedSearch : Eichler_1994_Mol.Pharmacol_45_335 |
| PubMedID: 8114681 |
| Title : Acetylcholinesterase peripheral anionic site degeneracy conferred by amino acid arrays sharing a common core - Barak_1994_J.Biol.Chem_269_6296 |
| Author(s) : Barak D , Kronman C , Ordentlich A , Ariel N , Bromberg A , Marcus D , Lazar A , Velan B , Shafferman A |
| Ref : Journal of Biological Chemistry , 269 :6296 , 1994 |
| Abstract : Barak_1994_J.Biol.Chem_269_6296 |
| ESTHER : Barak_1994_J.Biol.Chem_269_6296 |
| PubMedSearch : Barak_1994_J.Biol.Chem_269_6296 |
| PubMedID: 8119978 |
| Title : Retardation of neuritic outgrowth and cytoskeletal changes accompany acetylcholinesterase inhibitor treatment in cultured rat dorsal root ganglion neurons - Dupree_1994_J.Neurosci.Res_39_567 |
| Author(s) : Dupree JL , Bigbee JW |
| Ref : Journal of Neuroscience Research , 39 :567 , 1994 |
| Abstract : Dupree_1994_J.Neurosci.Res_39_567 |
| ESTHER : Dupree_1994_J.Neurosci.Res_39_567 |
| PubMedSearch : Dupree_1994_J.Neurosci.Res_39_567 |
| PubMedID: 7891392 |
| Title : Degradation of acetylcholine in human airways: role of butyrylcholinesterase - Norel_1993_Br.J.Pharmacol_108_914 |
| Author(s) : Norel X , Angrisani M , Labat C , Gorenne I , Dulmet E , Rossi F , Brink C |
| Ref : British Journal of Pharmacology , 108 :914 , 1993 |
| Abstract : Norel_1993_Br.J.Pharmacol_108_914 |
| ESTHER : Norel_1993_Br.J.Pharmacol_108_914 |
| PubMedSearch : Norel_1993_Br.J.Pharmacol_108_914 |
| PubMedID: 8485630 |
| Title : Binding of 125I-fasciculin to rat brain acetylcholinesterase. The complex still binds diisopropyl fluorophosphate - Marchot_1993_J.Biol.Chem_268_12458 |
| Author(s) : Marchot P , Khelif A , Ji YH , Mansuelle P , Bougis PE |
| Ref : Journal of Biological Chemistry , 268 :12458 , 1993 |
| Abstract : Marchot_1993_J.Biol.Chem_268_12458 |
| ESTHER : Marchot_1993_J.Biol.Chem_268_12458 |
| PubMedSearch : Marchot_1993_J.Biol.Chem_268_12458 |
| PubMedID: 8509385 |
| Title : Substrate inhibition of acetylcholinesterase: residues affecting signal transduction from the surface to the catalytic center - Shafferman_1992_EMBO.J_11_3561 |
| Author(s) : Shafferman A , Velan B , Ordentlich A , Kronman C , Grosfeld H , Leitner M , Flashner Y , Cohen S , Barak D , Ariel N |
| Ref : EMBO Journal , 11 :3561 , 1992 |
| Abstract : Shafferman_1992_EMBO.J_11_3561 |
| ESTHER : Shafferman_1992_EMBO.J_11_3561 |
| PubMedSearch : Shafferman_1992_EMBO.J_11_3561 |
| PubMedID: 1396557 |
| Title : Different functional pools of acetylcholinesterase induce changes in rat locus coeruleus noradrenaline metabolism - Abo_1992_Neurosci.Lett_141_111 |
| Author(s) : Abo V , Viera L , Dajas F |
| Ref : Neuroscience Letters , 141 :111 , 1992 |
| Abstract : Abo_1992_Neurosci.Lett_141_111 |
| ESTHER : Abo_1992_Neurosci.Lett_141_111 |
| PubMedSearch : Abo_1992_Neurosci.Lett_141_111 |
| PubMedID: 1508391 |
| Title : The effect of pesticides on carp (Cyprinus carpio L). Acetylcholinesterase and its biochemical characterization - Szabo_1992_Ecotoxicol.Environ.Saf_23_39 |
| Author(s) : Szabo A , Nemcsok J , Asztalos B , Rakonczay Z , Kasa P , Hieu LH |
| Ref : Ecotoxicology & Environmental Safety , 23 :39 , 1992 |
| Abstract : Szabo_1992_Ecotoxicol.Environ.Saf_23_39 |
| ESTHER : Szabo_1992_Ecotoxicol.Environ.Saf_23_39 |
| PubMedSearch : Szabo_1992_Ecotoxicol.Environ.Saf_23_39 |
| PubMedID: 1375147 |
| Title : Preferential inhibition of acetylcholinesterase molecular forms in rat brain - Ogane_1992_Neurochem.Res_17_489 |
| Author(s) : Ogane N , Giacobini E , Messamore E |
| Ref : Neurochemical Research , 17 :489 , 1992 |
| Abstract : Ogane_1992_Neurochem.Res_17_489 |
| ESTHER : Ogane_1992_Neurochem.Res_17_489 |
| PubMedSearch : Ogane_1992_Neurochem.Res_17_489 |
| PubMedID: 1528356 |
| Title : Cholinesterases in rabbit serum - Simeon_1988_Gen.Pharmacol_19_849 |
| Author(s) : Simeon-Rudolf V , Reiner E , Skrinjaric-Spoljar M , Krauthacker B |
| Ref : General Pharmacology , 19 :849 , 1988 |
| Abstract : Simeon_1988_Gen.Pharmacol_19_849 |
| ESTHER : Simeon_1988_Gen.Pharmacol_19_849 |
| PubMedSearch : Simeon_1988_Gen.Pharmacol_19_849 |
| PubMedID: 3229626 |
| Title : Polymorphism of pseudocholinesterase in Torpedo marmorata tissues: comparative study of the catalytic and molecular properties of this enzyme with acetylcholinesterase - Toutant_1985_J.Neurochem_44_580 |
| Author(s) : Toutant JP , Massoulie J , Bon S |
| Ref : Journal of Neurochemistry , 44 :580 , 1985 |
| Abstract : Toutant_1985_J.Neurochem_44_580 |
| ESTHER : Toutant_1985_J.Neurochem_44_580 |
| PubMedSearch : Toutant_1985_J.Neurochem_44_580 |
| PubMedID: 2578181 |
| Title : Acetylcholinesterase activity of Xenopus laevis oocytes - Gundersen_1983_Neurosci_10_1487 |
| Author(s) : Gundersen CB , Miledi R |
| Ref : Neuroscience , 10 :1487 , 1983 |
| Abstract : Gundersen_1983_Neurosci_10_1487 |
| ESTHER : Gundersen_1983_Neurosci_10_1487 |
| PubMedSearch : Gundersen_1983_Neurosci_10_1487 |
| PubMedID: 6664498 |
| Title : Serotonin-sensitive aryl acylamidase activity of platelet acetylcholinesterase - Majumdar_1982_Biochem.Pharmacol_31_2319 |
| Author(s) : Majumdar R , George ST , Balasubramanian AS |
| Ref : Biochemical Pharmacology , 31 :2319 , 1982 |
| Abstract : Majumdar_1982_Biochem.Pharmacol_31_2319 |
| ESTHER : Majumdar_1982_Biochem.Pharmacol_31_2319 |
| PubMedSearch : Majumdar_1982_Biochem.Pharmacol_31_2319 |
| PubMedID: 7126246 |
| Title : Acetylcholinesterase and butyrylcholinesterase activity in the atria of the heart of adult albino rats - Slavikova_1982_Physiol.Bohemoslov_31_407 |
| Author(s) : Slavikova J , Vlk J , Hlavickova V |
| Ref : Physiol Bohemoslov , 31 :407 , 1982 |
| Abstract : Slavikova_1982_Physiol.Bohemoslov_31_407 |
| ESTHER : Slavikova_1982_Physiol.Bohemoslov_31_407 |
| PubMedSearch : Slavikova_1982_Physiol.Bohemoslov_31_407 |
| PubMedID: 6217470 |
| Title : The identity of the serotonin-sensitive aryl acylamidase with acetylcholinesterase from human erythrocytes, sheep basal ganglia and electric eel - George_1980_Eur.J.Biochem_111_511 |
| Author(s) : George ST , Balasubramanian AS |
| Ref : European Journal of Biochemistry , 111 :511 , 1980 |
| Abstract : George_1980_Eur.J.Biochem_111_511 |
| ESTHER : George_1980_Eur.J.Biochem_111_511 |
| PubMedSearch : George_1980_Eur.J.Biochem_111_511 |
| PubMedID: 7460914 |