Azinphos-Ethyl
General
Type : Organophosphate,Sulfur Compound,Organothiophosphate,Triazine
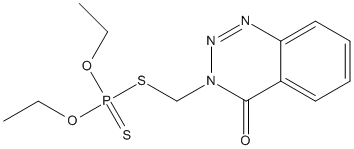
Chemical_Nomenclature : 3-(diethoxyphosphinothioylsulfanylmethyl)-1,2,3-benzotriazin-4-one
Canonical SMILES : CCOP(=S)(OCC)SCN1C(=O)C2=CC=CC=C2N=N1
InChI : InChI=1S\/C12H16N3O3PS2\/c1-3-17-19(20,18-4-2)21-9-15-12(16)10-7-5-6-8-11(10)13-14-15\/h5-8H,3-4,9H2,1-2H3
InChIKey : RQVGAIADHNPSME-UHFFFAOYSA-N
Other name(s) : Crysthion,Azinos,Bionex,Gutex,Athyl-gusathion,Azinophos-ethyl,Azinophosethyl,Ethyl azinphos,Ethylazinphos,Cotnion-ethyl,Ethyl Gusathion,O,O-diethyl S-[(4-oxo-1,2,3-benzotriazin-3(4H)-yl)methyl] phosphorodithioate,O,O-diethylphosphorodithioate S-ester with 3-mercaptomethyl-1,2,3-benzotriazin-4(3H)-one,Ethyl Guthion
MW : 333.36
Formula : C11H16N3O3PS2
CAS_number : 2642-71-9
PubChem : 17531
UniChem : RQVGAIADHNPSME-UHFFFAOYSA-N
IUPHAR :
Wikipedia : Azinphos-ethyl
Target
References (3)
| Title : Ethylazinphos interaction with membrane lipid organization induces increase of proton permeability and impairment of mitochondrial bioenergetic functions - Videira_2001_Toxicol.Appl.Pharmacol_175_209 |
| Author(s) : Videira RA , Antunes-Madeira MC , Madeira VM |
| Ref : Toxicol Appl Pharmacol , 175 :209 , 2001 |
| Abstract : Videira_2001_Toxicol.Appl.Pharmacol_175_209 |
| ESTHER : Videira_2001_Toxicol.Appl.Pharmacol_175_209 |
| PubMedSearch : Videira_2001_Toxicol.Appl.Pharmacol_175_209 |
| PubMedID: 11559019 |
| Title : Determination of organophosphorous and carbamate insecticides by flow injection analysis - Kumaran_1992_Anal.Biochem_200_187 |
| Author(s) : Kumaran S , Tran-Minh C |
| Ref : Analytical Biochemistry , 200 :187 , 1992 |
| Abstract : Kumaran_1992_Anal.Biochem_200_187 |
| ESTHER : Kumaran_1992_Anal.Biochem_200_187 |
| PubMedSearch : Kumaran_1992_Anal.Biochem_200_187 |
| PubMedID: 1595894 |
| Title : Gas-liquid chromatographic determination of organophosphorus insecticide residues in fruits and vegetables - Ferreira_1980_J.Assoc.Off.Anal.Chem_63_517 |
| Author(s) : Ferreira JR , Silva Fernandes AM |
| Ref : J Assoc Off Analytical Chemistry , 63 :517 , 1980 |
| Abstract : Ferreira_1980_J.Assoc.Off.Anal.Chem_63_517 |
| ESTHER : Ferreira_1980_J.Assoc.Off.Anal.Chem_63_517 |
| PubMedSearch : Ferreira_1980_J.Assoc.Off.Anal.Chem_63_517 |
| PubMedID: 7430038 |