Ambenonium
inhibitor of cholinesterase of longer duration than neostigmine. Oral treatment of myasthenia gravis
General
Type : Bisquaternary,Drug
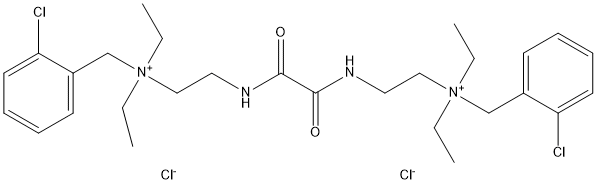
Chemical_Nomenclature : N,N'-bis(2-chlorobenzyldiethylammoniummethyl)oxamide dichloride Oxalbis(iminomethylene)bis(o-chlorobenzyl)diethylammonium chloride
Canonical SMILES : CC[N+](CC)(CCNC(=O)C(=O)NCC[N+](CC)(CC)CC1=CC=CC=C1Cl)CC2=CC=CC=C2Cl
InChI : InChI=1S\/C28H40Cl2N4O2\/c1-5-33(6-2,21-23-13-9-11-15-25(23)29)19-17-31-27(35)28(36)32-18-20-34(7-3,8-4)22-24-14-10-12-16-26(24)30\/h9-16H,5-8,17-22H2,1-4H3\/p+2
InChIKey : OMHBPUNFVFNHJK-UHFFFAOYSA-P
Other name(s) : Mytelase,Ambenonium chloride,Oxamizil,Oxazylum,Misuran,Mysuran,Oksazil,Oxazil,Oxazyl,Oxaz,WIN8077
MW : 608.47
Formula : C28H42Cl4N4O2
CAS_number : 115-79-7
PubChem : 2131
UniChem : OMHBPUNFVFNHJK-UHFFFAOYSA-P
IUPHAR :
Wikipedia : Ambenonium_chloride
Target
References (7)
| Title : The rate of thermal inactivation of Torpedo acetylcholinesterase is not reduced in the C231S mutant - Wilson_1996_FEBS.Lett_379_161 |
| Author(s) : Wilson EJ , Massoulie J , Bon S , Rosenberry TL |
| Ref : FEBS Letters , 379 :161 , 1996 |
| Abstract : Wilson_1996_FEBS.Lett_379_161 |
| ESTHER : Wilson_1996_FEBS.Lett_379_161 |
| PubMedSearch : Wilson_1996_FEBS.Lett_379_161 |
| PubMedID: 8635584 |
| Title : Quaternary nitrogen compounds affect carnitine distribution in rats. Particular emphasis on edrophonium - Pessotto_1996_Biochim.Biophys.Acta_1299_245 |
| Author(s) : Pessotto P , Liberati R , Petrella O , Hulsmann WC |
| Ref : Biochimica & Biophysica Acta , 1299 :245 , 1996 |
| Abstract : Pessotto_1996_Biochim.Biophys.Acta_1299_245 |
| ESTHER : Pessotto_1996_Biochim.Biophys.Acta_1299_245 |
| PubMedSearch : Pessotto_1996_Biochim.Biophys.Acta_1299_245 |
| PubMedID: 8555270 |
| Title : Comparative pharmacokinetics of four cholinesterase inhibitors in rats - Yamamoto_1995_Biol.Pharm.Bull_18_1292 |
| Author(s) : Yamamoto K , Sawada Y , Iga T |
| Ref : Biol Pharm Bull , 18 :1292 , 1995 |
| Abstract : Yamamoto_1995_Biol.Pharm.Bull_18_1292 |
| ESTHER : Yamamoto_1995_Biol.Pharm.Bull_18_1292 |
| PubMedSearch : Yamamoto_1995_Biol.Pharm.Bull_18_1292 |
| PubMedID: 8845827 |
| Title : Sensitive determination of ambenonium in plasma using inhibitory activity to acetylcholinesterase - Yamamoto_1993_Biol.Pharm.Bull_16_280 |
| Author(s) : Yamamoto K , Sawada Y , Iga T |
| Ref : Biol Pharm Bull , 16 :280 , 1993 |
| Abstract : Yamamoto_1993_Biol.Pharm.Bull_16_280 |
| ESTHER : Yamamoto_1993_Biol.Pharm.Bull_16_280 |
| PubMedSearch : Yamamoto_1993_Biol.Pharm.Bull_16_280 |
| PubMedID: 8364473 |
| Title : Ambenonium is a rapidly reversible noncovalent inhibitor of acetylcholinesterase, with one of the highest known affinities - Hodge_1992_Mol.Pharmacol_41_937 |
| Author(s) : Hodge AS , Humphrey DR , Rosenberry TL |
| Ref : Molecular Pharmacology , 41 :937 , 1992 |
| Abstract : Hodge_1992_Mol.Pharmacol_41_937 |
| ESTHER : Hodge_1992_Mol.Pharmacol_41_937 |
| PubMedSearch : Hodge_1992_Mol.Pharmacol_41_937 |
| PubMedID: 1588924 |
| Title : Inhibition of human motor endplate cholinesterase by anticholinesterase compounds - Fujii_1982_Acta.Med.Okayama_36_229 |
| Author(s) : Fujii M , Namba T |
| Ref : Acta Med Okayama , 36 :229 , 1982 |
| Abstract : Fujii_1982_Acta.Med.Okayama_36_229 |
| ESTHER : Fujii_1982_Acta.Med.Okayama_36_229 |
| PubMedSearch : Fujii_1982_Acta.Med.Okayama_36_229 |
| PubMedID: 7113748 |