ABX-1431
Monoacylglycerol lipase (human-MGLL) inhibitor (IC50 14 nM) .Increases brain 2-AG concentrations, and suppresses pain behavior. ABX-1431 has been shown to reduce tics in patients with Tourette syndrome
General
Type : Piperazine,Carbamate,Pyrrolidine,Trifluoro
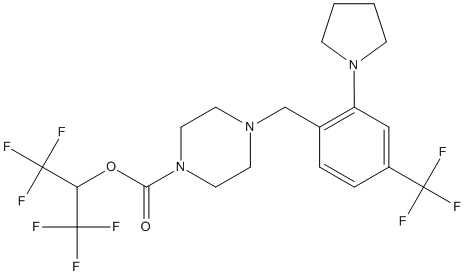
Chemical_Nomenclature : 1,1,1,3,3,3-hexafluoropropan-2-yl 4-[[2-pyrrolidin-1-yl-4-(trifluoromethyl)phenyl]methyl]piperazine-1-carboxylate
Canonical SMILES : C1CCN(C1)C2=C(C=CC(=C2)C(F)(F)F)CN3CCN(CC3)C(=O)OC(C(F)(F)F)C(F)(F)F
InChI : InChI=1S\/C20H22F9N3O2\/c21-18(22,23)14-4-3-13(15(11-14)31-5-1-2-6-31)12-30-7-9-32(10-8-30)17(33)34-16(19(24,25)26)20(27,28)29\/h3-4,11,16H,1-2,5-10,12H2
InChIKey : SQZJGTOZFRNWCX-UHFFFAOYSA-N
Other name(s) : CHEMBL3945728,SCHEMBL15100632,GTPL10062,Compound 28,Lu AG06466
MW : 507.4
Formula : C20H22F9N3O2
CAS_number : 1446817-84-0
PubChem : 71657619
UniChem : SQZJGTOZFRNWCX-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : ABX-1431 ligand of proteins in family: Monoglyceridelipase_lysophospholip
Stucture :
Protein : human-MGLL
References (5)
| Title : The Monoacylglycerol Lipase Inhibitor ABX-1431 Does Not Improve Alcoholic Liver Disease - Lucitti_2023_Cannabis.Cannabinoid.Res__ |
| Author(s) : Lucitti JL , Laudermilk LT , Amato GS , Maitra R |
| Ref : Cannabis Cannabinoid Res , : , 2023 |
| Abstract : Lucitti_2023_Cannabis.Cannabinoid.Res__ |
| ESTHER : Lucitti_2023_Cannabis.Cannabinoid.Res__ |
| PubMedSearch : Lucitti_2023_Cannabis.Cannabinoid.Res__ |
| PubMedID: 37253145 |
| Title : Monoacylglycerol Lipase Inhibition in Tourette Syndrome: A 12-Week, Randomized, Controlled Study - Muller-Vahl_2021_Mov.Disord__ |
| Author(s) : Muller-Vahl KR , Fremer C , Beals C , Ivkovic J , Loft H , Schindler C |
| Ref : Movement Disordersord , : , 2021 |
| Abstract : Muller-Vahl_2021_Mov.Disord__ |
| ESTHER : Muller-Vahl_2021_Mov.Disord__ |
| PubMedSearch : Muller-Vahl_2021_Mov.Disord__ |
| PubMedID: 34117788 |
| Title : Monoacylglycerol lipase inhibitors: modulators for lipid metabolism in cancer malignancy, neurological and metabolic disorders - Deng_2020_Acta.Pharm.Sin.B_10_582 |
| Author(s) : Deng H , Li W |
| Ref : Acta Pharm Sin B , 10 :582 , 2020 |
| Abstract : Deng_2020_Acta.Pharm.Sin.B_10_582 |
| ESTHER : Deng_2020_Acta.Pharm.Sin.B_10_582 |
| PubMedSearch : Deng_2020_Acta.Pharm.Sin.B_10_582 |
| PubMedID: 32322464 |
| Title : Activity-Based Protein Profiling Delivers Selective Drug Candidate ABX-1431, a Monoacylglycerol Lipase Inhibitor, To Control Lipid Metabolism in Neurological Disorders - Jiang_2018_J.Med.Chem_61_9059 |
| Author(s) : Jiang M , van der Stelt M |
| Ref : Journal of Medicinal Chemistry , 61 :9059 , 2018 |
| Abstract : Jiang_2018_J.Med.Chem_61_9059 |
| ESTHER : Jiang_2018_J.Med.Chem_61_9059 |
| PubMedSearch : Jiang_2018_J.Med.Chem_61_9059 |
| PubMedID: 30354159 |
| Title : Identification of ABX-1431, a Selective Inhibitor of Monoacylglycerol Lipase and Clinical Candidate for Treatment of Neurological Disorders - Cisar_2018_J.Med.Chem_61_9062 |
| Author(s) : Cisar JS , Weber OD , Clapper JR , Blankman JL , Henry CL , Simon GM , Alexander JP , Jones TK , Ezekowitz RAB , O'Neill GP , Grice CA |
| Ref : Journal of Medicinal Chemistry , 61 :9062 , 2018 |
| Abstract : Cisar_2018_J.Med.Chem_61_9062 |
| ESTHER : Cisar_2018_J.Med.Chem_61_9062 |
| PubMedSearch : Cisar_2018_J.Med.Chem_61_9062 |
| PubMedID: 30067909 |