6RUA-3a
IC50 60nM for human BChE residual activity of 93% mAChE or 94% human AChE. Compound 3A of the paper Pajk et al. derived from inhibitor 5DYW-5hf descibed in Kosak et al. 2016
General
Type : Sulfur Compound,Sulfonamide,Coumarin,Piperidine,Carboxamide,Naphthalen
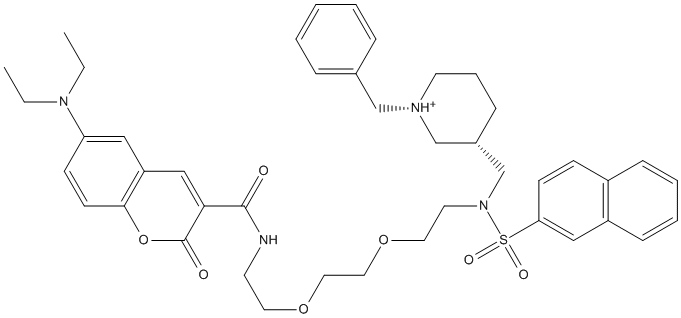
Chemical_Nomenclature : N-(2-(2-(2-(N-((1-Benzylpiperidin-3-yl)methyl)naphthalene-2-sulfonamido)ethoxy)ethoxy)ethyl)-7-(diethylamino)-2-oxo-2H-chromene-3-carboxamide || [(4aR,6R,8aR)-6-[(R)-[2-[2-[2-[[(3R)-1-benzylpiperidin-3-yl]methyl-naphthalen-2-ylsulfonylamino]ethoxy]ethoxy]ethylamino]-hydroxymethyl]-7-oxo-1,3,4,4a,5,6,8,8a-octahydronaphthalen-2-ylidene]-diethylazanium
Canonical SMILES : C(CN([S](=O)(=O)C1=CC=C2C(=C1)C=CC=C2)C[C@H]3CCC[N+](C3)CC4=CC=CC=C4)OCCOCCOCCNC(=O)C5=CC6C(OC5=O)C=C(C=C6)N(CC)CC || CC[N+](=C1CCC2CC(C(=O)CC2C1)C(NCCOCCOCCN(CC3CCCN(C3)CC4=CC=CC=C4)S(=O)(=O)C5=CC6=CC=CC=C6C=C5)O)CC
InChI : InChI=1S\/C45H58N4O8S\/c1-3-48(4-2)40-18-16-39-30-42(45(51)57-43(39)31-40)44(50)46-20-23-54-25-27-56-28-26-55-24-22-49(58(52,53)41-19-17-37-14-8-9-15-38(37)29-41)34-36-13-10-21-47(33-36)32-35-11-6-5-7-12-35\/h5-9,11-12,14-19,29-31,36,39,43H,3-4,10,13,20-28,32-34H2,1-2H3,(H,46,50)\/p+1\/t36-,39?,43?\/m0\/s1 || InChI=1S\/C44H63N4O6S\/c1-3-47(4-2)40-18-16-38-29-42(43(49)30-39(38)27-40)44(50)45-20-23-53-25-26-54-24-22-48(55(51,52)41-19-17-36-14-8-9-15-37(36)28-41)33-35-13-10-21-46(32-35)31-34-11-6-5-7-12-34\/h5-9,11-12,14-15,17,19,28,35,38-39,42,44-45,50H,3-4,10,13,16,18,20-27,29-33H2,1-2H3\/q+1\/t35-,38-,39-,42+,44-\/m1\/s1
InChIKey : XQMALPZSPCXNAF-DZRGVNRPSA-O || NSZMSICZTXQEMY-VMCDXIQDSA-N
Other name(s) : KJT
MW : 816.043 || 776
Formula : C45H59N4O8S || C44H63N4O6S+
CAS_number :
PubChem : 145946045
UniChem : XQMALPZSPCXNAF-DZRGVNRPSA-O || NSZMSICZTXQEMY-VMCDXIQDSA-N
IUPHAR :
Wikipedia :
Target
Families : 6RUA-3a ligand of proteins in family: BCHE
Stucture : 6RUA Structure of recombinant human butyrylcholinesterase in complex with a coumarin-based fluorescent probe linked to sulfonamide type inhibitor
Protein : human-BCHE
References (1)
| Title : Development of potent reversible selective inhibitors of butyrylcholinesterase as fluorescent probes - Pajk_2020_J.Enzyme.Inhib.Med.Chem_35_498 |
| Author(s) : Pajk S , Knez D , Kosak U , Zorovic M , Brazzolotto X , Coquelle N , Nachon F , Colletier JP , Zivin M , Stojan J , Gobec S |
| Ref : J Enzyme Inhib Med Chem , 35 :498 , 2020 |
| Abstract : Pajk_2020_J.Enzyme.Inhib.Med.Chem_35_498 |
| ESTHER : Pajk_2020_J.Enzyme.Inhib.Med.Chem_35_498 |
| PubMedSearch : Pajk_2020_J.Enzyme.Inhib.Med.Chem_35_498 |
| PubMedID: 31914836 |
| Gene_locus related to this paper: human-BCHE |