6F7Q-cpd11
N-Propargyliperidines with low nanomolar inhibition of butyrylcholinesterase (Ki = 1.09+/-0.12 nM). In addition, compound 11 show metal-chelating properties, and reduce the redox activity of chelated Cu2+ ions in a Cu-ascorbate redox system good antioxidant activity as determined by the DPPH assay
General
Type : Multitarget,Antioxidant,Piperidine,Quinoline,Metal ion chelator,Inden,Carboxamide
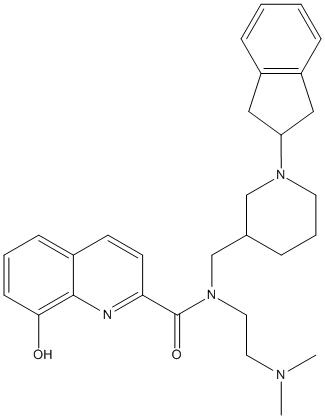
Chemical_Nomenclature : 4.5.4.(+\/-)-N-((1-(2,3-dihydro-1H-inden-2-yl)piperidin-3-yl)methyl)-N-(2-(dimethylamino)ethyl)-8-hydroxyquinoline-2-carboxamide
Canonical SMILES : CN(C)CCN(CC1CCCN(C1)C3CC2=CC=CC=C2C3)C(=O)C5=NC4=C(C=CC=C4O)C=C5
InChI : InChI=1S\/C29H36N4O2.C2H6\/c1-31(2)15-16-33(29(35)26-13-12-22-10-5-11-27(34)28(22)30-26)20-21-7-6-14-32(19-21)25-17-23-8-3-4-9-24(23)18-25\;1-2\/h3-5,8-13,21,25,34H,6-7,14-20H2,1-2H3\;1-2H3
InChIKey : JJYUCJIZTWRMMC-UHFFFAOYSA-N
Other name(s) :
MW : 472.62
Formula : C29H36N4O2
CAS_number :
PubChem :
UniChem : JJYUCJIZTWRMMC-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : 6F7Q-cpd11 ligand of proteins in family: BCHE
Stucture : 6F7Q Human Butyrylcholinesterase complexed with N-Propargyliperidines
Protein : human-BCHE
References (1)
| Title : Multi-target-directed ligands for treating Alzheimer's disease: Butyrylcholinesterase inhibitors displaying antioxidant and neuroprotective activities - Knez_2018_Eur.J.Med.Chem_156_598 |
| Author(s) : Knez D , Coquelle N , Pislar A , Zakelj S , Jukic M , Sova M , Mravljak J , Nachon F , Brazzolotto X , Kos J , Colletier JP , Gobec S |
| Ref : Eur Journal of Medicinal Chemistry , 156 :598 , 2018 |
| Abstract : Knez_2018_Eur.J.Med.Chem_156_598 |
| ESTHER : Knez_2018_Eur.J.Med.Chem_156_598 |
| PubMedSearch : Knez_2018_Eur.J.Med.Chem_156_598 |
| PubMedID: 30031971 |
| Gene_locus related to this paper: human-BCHE |