5AP
General
Type : Pyrimidine
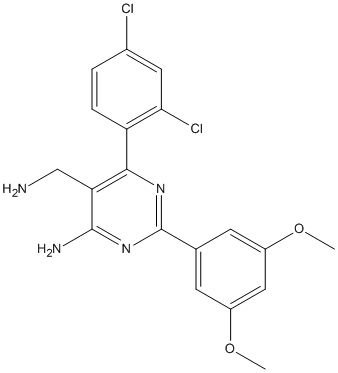
Chemical_Nomenclature : 5-(aminomethyl)-6-(2,4-dichlorophenyl)-2-(3,5-dimethoxyphenyl)pyrimidin-4-amine
Canonical SMILES : COC1=CC(=CC(=C1)C2=NC(=C(C(=N2)N)CN)C3=C(C=C(C=C3)Cl)Cl)OC
InChI : InChI=1S\/C19H18Cl2N4O2\/c1-26-12-5-10(6-13(8-12)27-2)19-24-17(15(9-22)18(23)25-19)14-4-3-11(20)7-16(14)21\/h3-8H,9,22H2,1-2H3,(H2,23,24,25)
InChIKey : RCJFINNFYUNFGH-UHFFFAOYSA-N
Other name(s) : Aminomethylpyrimidine 1e,AC1L9LYJ,CHEMBL34062
MW : 405.27
Formula : C19H18Cl2N4O2
CAS_number :
PubChem : 448436
UniChem : RCJFINNFYUNFGH-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : 5AP ligand of proteins in family: DPP4N_Peptidase_S9
Stucture : 1RWQ Human Dipeptidyl peptidase IV in complex with 5-aminomethyl-6-(2,4-dichloro-phenyl)-2-(3,5-dimethoxy-phenyl)-pyrimidin-4-amine
Protein : human-DPP4
References (1)
| Title : Aminomethylpyrimidines as novel DPP-IV inhibitors: a 10(5)-fold activity increase by optimization of aromatic substituents - Peters_2004_Bioorg.Med.Chem.Lett_14_1491 |
| Author(s) : Peters JU , Weber S , Kritter S , Weiss P , Wallier A , Boehringer M , Hennig M , Kuhn B , Loeffler BM |
| Ref : Bioorganic & Medicinal Chemistry Lett , 14 :1491 , 2004 |
| Abstract : Peters_2004_Bioorg.Med.Chem.Lett_14_1491 |
| ESTHER : Peters_2004_Bioorg.Med.Chem.Lett_14_1491 |
| PubMedSearch : Peters_2004_Bioorg.Med.Chem.Lett_14_1491 |
| PubMedID: 15006388 |
| Gene_locus related to this paper: human-DPP4 |