5ALU-HD2
IC50 22 nM.human-EPHX2. 5ALU-HD2 is also inhibitor of 11-beta-Hydroxysteroid dehydrogenase 1 (11beta-HSD1) has been a target of intensive research efforts across the pharmaceutical industry, due to its potential for the treatment of type II diabetes and other elements of the metabolic syndrome Human HTRF in vitro potency: 21nM Murine HTRF in vitro potency:32 nM Murine in vivi (PK/PD) potency: 50nM. It has been cristallized with 11beta-HSD1 pdb:4BB5 Goldberg et al.
General
Type : Adamantyl,Urea derivative,Carboxamide,Pyrimidine,Not A\/B H target
Chemical_Nomenclature : 4-cyclopentyl-N-[(1R,3S)-5-hydroxy-2-adamantyl]-2-[[(3S)-oxolan-3-yl]amino]pyrimidine-5-carboxamide
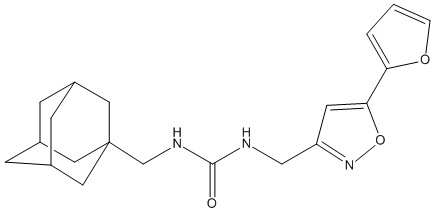
Canonical SMILES : C1CCC(C1)C2=NC(=NC=C2C(=O)NC3C4CC5CC3CC(C5)(C4)O)NC6CCOC6
InChI : InChI=1S\/C24H34N4O3\/c29-22(27-20-16-7-14-8-17(20)11-24(30,9-14)10-16)19-12-25-23(26-18-5-6-31-13-18)28-21(19)15-3-1-2-4-15\/h12,14-18,20,30H,1-11,13H2,(H,27,29)(H,25,26,28)\/t14?,16-,17+,18-,20?,24?\/m0\/s1 || InChI=1S\/C24H34N4O3\/c29-22(27-20-16-7-14-8-17(20)11-24(30,9-14)10-16)19-12-25-23(26-18-5-6-31-13-18)28-21(19)15-3-1-2-4-15\/h12,14-18,20,30H,1-11,13H2,(H,27,29)(H,25,26,28)\/t14-,16-,17+,18-,20-,24-\/m0\/s1
InChIKey : VMTZDIHMLVWUQZ-XANBXHNASA-N || VMTZDIHMLVWUQZ-GXLRSYGRSA-N
Other name(s) : HD2,4-cyclopentyl-N-[(1R,2s,3S,5s,7s)-5-hydroxytricyclo[3.3.1.1~3,7~]dec-2-yl]-2-[(3S)-tetrahydrofuran-3-ylamino]pyrimidine-5-carboxamide
MW : 426.6
Formula : C24H34N4O3
CAS_number :
PubChem : 70678489
UniChem : VMTZDIHMLVWUQZ-XANBXHNASA-N || VMTZDIHMLVWUQZ-GXLRSYGRSA-N
IUPHAR :
Wikipedia :
Target
Families : 5ALU-HD2 ligand of proteins in family: Epoxide_hydrolase
Stucture : 5ALU ligand complex structure of human soluble epoxide hydrolase
Protein : human-EPHX2
References (2)
| Title : Successful generation of structural information for fragment-based drug discovery - Oster_2015_Drug.Discov.Today_20_1104 |
| Author(s) : Oster L , Tapani S , Xue Y , Kack H |
| Ref : Drug Discov Today , 20 :1104 , 2015 |
| Abstract : Oster_2015_Drug.Discov.Today_20_1104 |
| ESTHER : Oster_2015_Drug.Discov.Today_20_1104 |
| PubMedSearch : Oster_2015_Drug.Discov.Today_20_1104 |
| PubMedID: 25931264 |
| Gene_locus related to this paper: human-EPHX2 |
| Title : Free-Wilson and structural approaches to co-optimizing human and rodent isoform potency for 11beta-hydroxysteroid dehydrogenase type 1 (11beta-HSD1) inhibitors - Goldberg_2012_J.Med.Chem_55_10652 |
| Author(s) : Goldberg FW , Leach AG , Scott JS , Snelson WL , Groombridge SD , Donald CS , Bennett SN , Bodin C , Gutierrez PM , Gyte AC |
| Ref : Journal of Medicinal Chemistry , 55 :10652 , 2012 |
| Abstract : Goldberg_2012_J.Med.Chem_55_10652 |
| ESTHER : Goldberg_2012_J.Med.Chem_55_10652 |
| PubMedSearch : Goldberg_2012_J.Med.Chem_55_10652 |
| PubMedID: 23153367 |