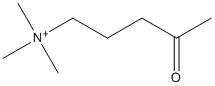
4-oxo-N,N,N-trimethylpentanaminium
General
Type : Trimethylammonium,Analogue of substrate
Chemical_Nomenclature : N,N,N-trimethyl-4-oxopentan-1-aminium
Canonical SMILES : CC(=O)CCC[N+](C)(C)C
InChI : InChI=1S\/C8H18NO\/c1-8(10)6-5-7-9(2,3)4\/h5-7H2,1-4H3\/q+1
InChIKey : UKCYTFTWLWVZSO-UHFFFAOYSA-N
Other name(s) : trimethyl-(4-oxopentyl)azanium,NWA,5-Tmap,KPTMA,4K-TMA,Ketopentyltrimethylammonium,Trimethyl(4-oxopentyl)ammonium,5-Trimethylammonio-2-pentanone,Trimethyl(4-oxopentyl)ammonium chloride,1-Pentanaminium, N,N,N-trimethyl-4-oxo-,4-Oxo-N,N,N-trimethylpentaminium chloride
MW : 144.23
Formula : C8H18NO
CAS_number : 25351-18-2 || 25351-37-5
PubChem : 91420
UniChem : UKCYTFTWLWVZSO-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : 4-oxo-N,N,N-trimethylpentanaminium ligand of proteins in family: ACHE
Stucture :
Protein : torca-ACHE
References (7)
| Title : Room temperature crystallography of human acetylcholinesterase bound to a substrate analogue 4K-TMA: Towards a neutron structure - Gerlits_2021_Curr.Res.Struct.Biol_3_206 |
| Author(s) : Gerlits O , Blakeley MP , Keen DA , Radic Z , Kovalevsky A |
| Ref : Current Research in Structural Biology , 3 :206 , 2021 |
| Abstract : Gerlits_2021_Curr.Res.Struct.Biol_3_206 |
| ESTHER : Gerlits_2021_Curr.Res.Struct.Biol_3_206 |
| PubMedSearch : Gerlits_2021_Curr.Res.Struct.Biol_3_206 |
| PubMedID: 34541552 |
| Gene_locus related to this paper: human-ACHE |
| Title : Shoot-and-Trap: use of specific x-ray damage to study structural protein dynamics by temperature-controlled cryo-crystallography - Colletier_2008_Proc.Natl.Acad.Sci.U.S.A_105_11742 |
| Author(s) : Colletier JP , Bourgeois D , Sanson B , Fournier D , Sussman JL , Silman I , Weik M |
| Ref : Proc Natl Acad Sci U S A , 105 :11742 , 2008 |
| Abstract : Colletier_2008_Proc.Natl.Acad.Sci.U.S.A_105_11742 |
| ESTHER : Colletier_2008_Proc.Natl.Acad.Sci.U.S.A_105_11742 |
| PubMedSearch : Colletier_2008_Proc.Natl.Acad.Sci.U.S.A_105_11742 |
| PubMedID: 18701720 |
| Gene_locus related to this paper: torca-ACHE |
| Title : Substrate and product trafficking through the active center gorge of acetylcholinesterase analyzed by crystallography and equilibrium binding - Bourne_2006_J.Biol.Chem_281_29256 |
| Author(s) : Bourne Y , Radic Z , Sulzenbacher G , Kim E , Taylor P , Marchot P |
| Ref : Journal of Biological Chemistry , 281 :29256 , 2006 |
| Abstract : Bourne_2006_J.Biol.Chem_281_29256 |
| ESTHER : Bourne_2006_J.Biol.Chem_281_29256 |
| PubMedSearch : Bourne_2006_J.Biol.Chem_281_29256 |
| PubMedID: 16837465 |
| Gene_locus related to this paper: mouse-ACHE |
| Title : Structural insights into substrate traffic and inhibition in acetylcholinesterase - Colletier_2006_EMBO.J_25_2746 |
| Author(s) : Colletier JP , Fournier D , Greenblatt HM , Stojan J , Sussman JL , Zaccai G , Silman I , Weik M |
| Ref : EMBO Journal , 25 :2746 , 2006 |
| Abstract : Colletier_2006_EMBO.J_25_2746 |
| ESTHER : Colletier_2006_EMBO.J_25_2746 |
| PubMedSearch : Colletier_2006_EMBO.J_25_2746 |
| PubMedID: 16763558 |
| Gene_locus related to this paper: torca-ACHE |
| Title : Involvement of deacylation in activation of substrate hydrolysis by Drosophila acetylcholinesterase - Brochier_2001_J.Biol.Chem_276_18296 |
| Author(s) : Brochier L , Pontie Y , Willson M , Estrada-Mondaca S , Czaplick J , Klaebe A , Fournier D |
| Ref : Journal of Biological Chemistry , 276 :18296 , 2001 |
| Abstract : Brochier_2001_J.Biol.Chem_276_18296 |
| ESTHER : Brochier_2001_J.Biol.Chem_276_18296 |
| PubMedSearch : Brochier_2001_J.Biol.Chem_276_18296 |
| PubMedID: 11278288 |
| Title : Reactions of 1-bromo-2-[14C]pinacolone with acetylcholinesterase from Torpedo nobiliana. Effects of 5-trimethylammonio-2-pentanone and diisopropyl fluorophosphate - Cohen_1989_Biochim.Biophys.Acta_997_167 |
| Author(s) : Cohen SG , Salih E , Solomon M , Howard S , Chishti SB , Cohen JB |
| Ref : Biochimica & Biophysica Acta , 997 :167 , 1989 |
| Abstract : Cohen_1989_Biochim.Biophys.Acta_997_167 |
| ESTHER : Cohen_1989_Biochim.Biophys.Acta_997_167 |
| PubMedSearch : Cohen_1989_Biochim.Biophys.Acta_997_167 |
| PubMedID: 2765553 |
| Title : Inactivation of acetylcholinesterase with a bretylium tosylate photoaffinity probe - Branchini_1986_Biochim.Biophys.Acta_884_135 |
| Author(s) : Branchini BR , Lajiness EJ |
| Ref : Biochimica & Biophysica Acta , 884 :135 , 1986 |
| Abstract : Branchini_1986_Biochim.Biophys.Acta_884_135 |
| ESTHER : Branchini_1986_Biochim.Biophys.Acta_884_135 |
| PubMedSearch : Branchini_1986_Biochim.Biophys.Acta_884_135 |
| PubMedID: 3768408 |