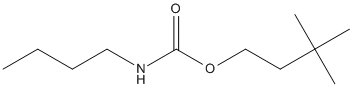
3,3-Dimethylbutyl-N-butylcarbamate
Inhibitor of ACHE but activator of BCHE
General
Type : Carbamate,Analogue of substrate
Chemical_Nomenclature : 3,3-dimethylbutyl N-butylcarbamate
Canonical SMILES : CCCCNC(=O)OCCC(C)(C)C
InChI : InChI=1S\/C11H23NO2\/c1-5-6-8-12-10(13)14-9-7-11(2,3)4\/h5-9H2,1-4H3,(H,12,13)
InChIKey : HMWVVNADQPDYDT-UHFFFAOYSA-N
Other name(s) : Butylcarbamic acid 3,3-dimethylbutyl ester,3,3-Dimethylbutyl-N-n-butylcarbamate
MW : 201.31
Formula : C11H23NO2
CAS_number :
PubChem : 102394631
UniChem : HMWVVNADQPDYDT-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : 3,3-Dimethylbutyl-N-butylcarbamate ligand of proteins in family: BCHE || ACHE
Stucture :
Protein :
References (2)
| Title : Activation mechanisms of butyrylcholinesterase by 2,4,6-trinitrotoluene, 3,3-dimethylbutyl-N-n-butylcarbamate, and 2-trimethylsilyl-ethyl-N-n-butylcarbamate - Chiou_2008_Appl.Biochem.Biotechnol_150_337 |
| Author(s) : Chiou SY , Wu YG , Lin G |
| Ref : Appl Biochem Biotechnol , 150 :337 , 2008 |
| Abstract : Chiou_2008_Appl.Biochem.Biotechnol_150_337 |
| ESTHER : Chiou_2008_Appl.Biochem.Biotechnol_150_337 |
| PubMedSearch : Chiou_2008_Appl.Biochem.Biotechnol_150_337 |
| PubMedID: 18563305 |
| Title : Substrate activation of butyrylcholinesterase and substrate inhibition of acetylcholinesterase by 3,3-dimethylbutyl-N-n-butylcarbamate and 2-trimethylsilyl-ethyl-N-n-butylcarbamate - Chiou_2007_J.Biochem.Mol.Toxicol_21_24 |
| Author(s) : Chiou SY , Wu YG , Lin YF , Lin LY , Lin G |
| Ref : J Biochem Mol Toxicol , 21 :24 , 2007 |
| Abstract : Chiou_2007_J.Biochem.Mol.Toxicol_21_24 |
| ESTHER : Chiou_2007_J.Biochem.Mol.Toxicol_21_24 |
| PubMedSearch : Chiou_2007_J.Biochem.Mol.Toxicol_21_24 |
| PubMedID: 17366539 |