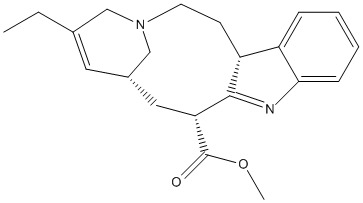
18-carboxymethoxy-cleaviminium
Cleaviminium intermediate
General
Type : Natural,Alkaloid
Chemical_Nomenclature : methyl (13R,15R)-17-ethyl-1,11-diazatetracyclo[13.3.1.04,12.05,10]nonadeca-4(12),5,7,9,16-pentaene-13-carboxylate
Canonical SMILES : CCC1=CC2CC(C3=C(CCN(C2)C1)C4=CC=CC=C4N3)C(=O)OC
InChI : InChI=1S\/C21H26N2O2\/c1-3-14-10-15-11-18(21(24)25-2)20-17(8-9-23(12-14)13-15)16-6-4-5-7-19(16)22-20\/h4-7,10,15,18,22H,3,8-9,11-13H2,1-2H3\/t15-,18+\/m0\/s1
InChIKey : DVKSNWMGQWIOHA-MAUKXSAKSA-N
Other name(s) : KJE,18beta-Carboxycleavamine methyl
MW : 338.4
Formula : C21H26N2O2
CAS_number : 26251-91-2
PubChem : 21598356
UniChem : DVKSNWMGQWIOHA-MAUKXSAKSA-N
IUPHAR :
Wikipedia :
Target
Families : 18-carboxymethoxy-cleaviminium ligand of proteins in family: Plant_carboxylesterase
Stucture : 6RT8 Structure of catharanthine synthase - an alpha-beta hydrolase from Catharanthus roseus with a cleaviminium intermediate bound
Protein : catro-CS
References (1)
| Title : Structural basis of cycloaddition in biosynthesis of iboga and aspidosperma alkaloids - Caputi_2020_Nat.Chem.Biol_16_383 |
| Author(s) : Caputi L , Franke J , Bussey K , Farrow SC , Vieira IJC , Stevenson CEM , Lawson DM , O'Connor SE |
| Ref : Nat Chemical Biology , 16 :383 , 2020 |
| Abstract : Caputi_2020_Nat.Chem.Biol_16_383 |
| ESTHER : Caputi_2020_Nat.Chem.Biol_16_383 |
| PubMedSearch : Caputi_2020_Nat.Chem.Biol_16_383 |
| PubMedID: 32066966 |
| Gene_locus related to this paper: catro-CS , catro-TS , tabib-a0a5b8x6s2 , tabib-CorS |