ST052207
highly selective BChE inhibitor
General
Type : Quinoline,Carbazol
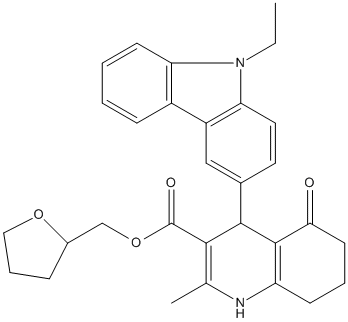
Chemical_Nomenclature : oxolan-2-ylmethyl 4-(9-ethylcarbazol-3-yl)-2-methyl-5-oxo-4,6,7,8-tetrahydro-1H-quinoline-3-carboxylate
Canonical SMILES : CCN1C2=C(C=C(C=C2)C3C4=C(CCCC4=O)NC(=C3C(=O)OCC5CCCO5)C)C6=CC=CC=C61
InChI : InChI=1S\/C30H32N2O4\/c1-3-32-24-11-5-4-9-21(24)22-16-19(13-14-25(22)32)28-27(30(34)36-17-20-8-7-15-35-20)18(2)31-23-10-6-12-26(33)29(23)28\/h4-5,9,11,13-14,16,20,28,31H,3,6-8,10,12,15,17H2,1-2H3
InChIKey : SHBFRAVLHSPWTB-UHFFFAOYSA-N
Other name(s) : ST052207,AC1MFAYO,BAS 02170398,CBMicro_047393,MixCom6_002337,Oprea1_577190
MW : 484.58
Formula : C30H32N2O4
CAS_number :
PubChem : 2891624
UniChem : SHBFRAVLHSPWTB-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : ST052207 ligand of proteins in family: BCHE
Stucture : 5K5E Discovery and structure-activity relationships of a highly selective Butyrylcholinesterase inhibitor by structure-based virtual screening (5JYW withdrawn)
Protein : human-BCHE
References (1)
| Title : Discovery and Structure-Activity Relationships of a Highly Selective Butyrylcholinesterase Inhibitor by Structure-Based Virtual Screening - Dighe_2016_J.Med.Chem_59_7683 |
| Author(s) : Dighe SN , Deora GS , De la Mora E , Nachon F , Chan S , Parat MO , Brazzolotto X , Ross BP |
| Ref : Journal of Medicinal Chemistry , 59 :7683 , 2016 |
| Abstract : Dighe_2016_J.Med.Chem_59_7683 |
| ESTHER : Dighe_2016_J.Med.Chem_59_7683 |
| PubMedSearch : Dighe_2016_J.Med.Chem_59_7683 |
| PubMedID: 27405689 |
| Gene_locus related to this paper: human-BCHE |