Isoparathion
position of sulfure and oxygene inverse of parathion
General
Type : Organophosphate,Sulfur Compound,Organothiophosphate,pNP
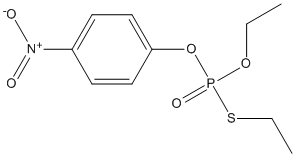
Chemical_Nomenclature : 1-(ethoxy-ethylsulfanyl-phosphoryl)oxy-4-nitro-benzene
Canonical SMILES : CCOP(=O)(OC1=CC=C(C=C1)[N+](=O)[O-])SCC
InChI : InChI=1S\/C10H14NO5PS\/c1-3-15-17(14,18-4-2)16-10-7-5-9(6-8-10)11(12)13\/h5-8H,3-4H2,1-2H3
InChIKey : BGWJTLLALYACOG-UHFFFAOYSA-N
Other name(s) : S-ethyl parathion
MW : 291.262
Formula : C10H14NO5PS
CAS_number :
PubChem : 11705
UniChem : BGWJTLLALYACOG-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (1)
| Title : Kinetic Evidence for Different Mechanisms of Acetylcholinesterase Inhibition by (1R)- and (1S)-Stereoisomers of Isomalathion - Jianmongkol_1999_Toxicol.Appl.Pharmacol_155_43 |
| Author(s) : Jianmongkol S , Marable BR , Berkman CE , Talley TT , Thompson CM , Richardson RJ |
| Ref : Toxicol Appl Pharmacol , 155 :43 , 1999 |
| Abstract : Jianmongkol_1999_Toxicol.Appl.Pharmacol_155_43 |
| ESTHER : Jianmongkol_1999_Toxicol.Appl.Pharmacol_155_43 |
| PubMedSearch : Jianmongkol_1999_Toxicol.Appl.Pharmacol_155_43 |
| PubMedID: 10036217 |