Cymserine
PEC (-)-N1-phenethylnorcymserine; BNC (-)-N1,n8-bisnorcymserine . BChE selectivity versus AChE (IC50 ratios) human: Cymserine 15-fold, PEC >5000-fold, BNC 110-fold; rat Cymserine 21-fold, PEC >3300-fold, BNC 150-fold
General
Type : Derivative of physostigmine-eserine,Carbamate,Indole
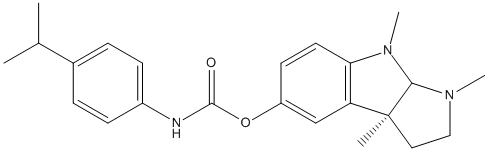
Chemical_Nomenclature : [(3aR,8bS)-3,4,8b-trimethyl-2,3a-dihydro-1H-pyrrolo[2,3-b]indol-7-yl] N-(4-propan-2-ylphenyl)carbamate
Canonical SMILES : CC(C)C1=CC=C(C=C1)NC(=O)OC2=CC3=C(C=C2)N(C4C3(CCN4C)C)C
InChI : InChI=1S\/C23H29N3O2\/c1-15(2)16-6-8-17(9-7-16)24-22(27)28-18-10-11-20-19(14-18)23(3)12-13-25(4)21(23)26(20)5\/h6-11,14-15,21H,12-13H2,1-5H3,(H,24,27)\/t21-,23+\/m1\/s1
InChIKey : NKJRRVBTMYRXRB-GGAORHGYSA-N
Other name(s) : 4-isopropylphenyserine,Cymserine.tartaric acid,D00VSE,D0O2OH
MW : 379.50
Formula : C23H29N3O2
CAS_number : 145209-39-8
PubChem : 9907847
UniChem : NKJRRVBTMYRXRB-GGAORHGYSA-N
IUPHAR :
Wikipedia : Cymserine
Target
References (13)
| Title : Inhibition of Butyrylcholinesterase with Fluorobenzylcymserine, An Experimental Alzheimer's Drug Candidate: Validation of Enzoinformatics Results by Classical and Innovative Enzyme Kinetic Analyses - Kamal_2017_CNS.Neurol.Disord.Drug.Targets_16_820 |
| Author(s) : Kamal MA , Shakil S , Nawaz MS , Yu QS , Tweedie D , Tan Y , Qu X , Greig NH |
| Ref : CNS Neurol Disord Drug Targets , 16 :820 , 2017 |
| Abstract : Kamal_2017_CNS.Neurol.Disord.Drug.Targets_16_820 |
| ESTHER : Kamal_2017_CNS.Neurol.Disord.Drug.Targets_16_820 |
| PubMedSearch : Kamal_2017_CNS.Neurol.Disord.Drug.Targets_16_820 |
| PubMedID: 28176640 |
| Title : Kinetics of Torpedo californica acetylcholinesterase inhibition by bisnorcymserine and crystal structure of the complex with its leaving group - Bartolucci_2012_Biochem.J_444_269 |
| Author(s) : Bartolucci C , Stojan J , Yu QS , Greig NH , Lamba D |
| Ref : Biochemical Journal , 444 :269 , 2012 |
| Abstract : Bartolucci_2012_Biochem.J_444_269 |
| ESTHER : Bartolucci_2012_Biochem.J_444_269 |
| PubMedSearch : Bartolucci_2012_Biochem.J_444_269 |
| PubMedID: 22390827 |
| Gene_locus related to this paper: torma-ACHE |
| Title : Tetrahydrofurobenzofuran cymserine, a potent butyrylcholinesterase inhibitor and experimental Alzheimer drug candidate, enzyme kinetic analysis - Kamal_2008_J.Neural.Transm.(Vienna)_115_889 |
| Author(s) : Kamal MA , Qu X , Yu QS , Tweedie D , Holloway HW , Li Y , Tan Y , Greig NH |
| Ref : J Neural Transm (Vienna) , 115 :889 , 2008 |
| Abstract : Kamal_2008_J.Neural.Transm.(Vienna)_115_889 |
| ESTHER : Kamal_2008_J.Neural.Transm.(Vienna)_115_889 |
| PubMedSearch : Kamal_2008_J.Neural.Transm.(Vienna)_115_889 |
| PubMedID: 18235987 |
| Title : Kinetics of human serum butyrylcholinesterase inhibition by a novel experimental Alzheimer therapeutic, dihydrobenzodioxepine cymserine - Kamal_2008_Neurochem.Res_33_745 |
| Author(s) : Kamal MA , Klein P , Luo W , Li Y , Holloway HW , Tweedie D , Greig NH |
| Ref : Neurochem Res , 33 :745 , 2008 |
| Abstract : Kamal_2008_Neurochem.Res_33_745 |
| ESTHER : Kamal_2008_Neurochem.Res_33_745 |
| PubMedSearch : Kamal_2008_Neurochem.Res_33_745 |
| PubMedID: 17985237 |
| Title : N1phenethyl-norcymserine, a selective butyrylcholinesterase inhibitor, increases acetylcholine release in rat cerebral cortex: a comparison with donepezil and rivastigmine - Cerbai_2007_Eur.J.Pharmacol_572_142 |
| Author(s) : Cerbai F , Giovannini MG , Melani C , Enz A , Pepeu G |
| Ref : European Journal of Pharmacology , 572 :142 , 2007 |
| Abstract : Cerbai_2007_Eur.J.Pharmacol_572_142 |
| ESTHER : Cerbai_2007_Eur.J.Pharmacol_572_142 |
| PubMedSearch : Cerbai_2007_Eur.J.Pharmacol_572_142 |
| PubMedID: 17643410 |
| Title : Kinetic analysis of the inhibition of human butyrylcholinesterase with cymserine - Kamal_2006_Biochim.Biophys.Acta_1760_200 |
| Author(s) : Kamal MA , Al-Jafari AA , Yu QS , Greig NH |
| Ref : Biochimica & Biophysica Acta , 1760 :200 , 2006 |
| Abstract : Kamal_2006_Biochim.Biophys.Acta_1760_200 |
| ESTHER : Kamal_2006_Biochim.Biophys.Acta_1760_200 |
| PubMedSearch : Kamal_2006_Biochim.Biophys.Acta_1760_200 |
| PubMedID: 16309845 |
| Title : Kinetics of human serum butyrylcholinesterase and its inhibition by a novel experimental Alzheimer therapeutic, bisnorcymserine - Kamal_2006_J.Alzheimers.Dis_10_43 |
| Author(s) : Kamal MA , Klein P , Yu QS , Tweedie D , Li Y , Holloway HW , Greig NH |
| Ref : J Alzheimers Dis , 10 :43 , 2006 |
| Abstract : Kamal_2006_J.Alzheimers.Dis_10_43 |
| ESTHER : Kamal_2006_J.Alzheimers.Dis_10_43 |
| PubMedSearch : Kamal_2006_J.Alzheimers.Dis_10_43 |
| PubMedID: 16988481 |
| Title : Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent - Greig_2005_Proc.Natl.Acad.Sci.U.S.A_102_17213 |
| Author(s) : Greig NH , Utsuki T , Ingram DK , Wang Y , Pepeu G , Scali C , Yu QS , Mamczarz J , Holloway HW , Giordano T , Chen D , Furukawa K , Sambamurti K , Brossi A , Lahiri DK |
| Ref : Proc Natl Acad Sci U S A , 102 :17213 , 2005 |
| Abstract : Greig_2005_Proc.Natl.Acad.Sci.U.S.A_102_17213 |
| ESTHER : Greig_2005_Proc.Natl.Acad.Sci.U.S.A_102_17213 |
| PubMedSearch : Greig_2005_Proc.Natl.Acad.Sci.U.S.A_102_17213 |
| PubMedID: 16275899 |
| Title : Intravenous butyrylcholinesterase administration and plasma and brain levels of cocaine and metabolites in rats - Carmona_2005_Eur.J.Pharmacol_517_186 |
| Author(s) : Carmona GN , Schindler CW , Greig NH , Holloway HW , Jufer RA , Cone EJ , Gorelick DA |
| Ref : European Journal of Pharmacology , 517 :186 , 2005 |
| Abstract : Carmona_2005_Eur.J.Pharmacol_517_186 |
| ESTHER : Carmona_2005_Eur.J.Pharmacol_517_186 |
| PubMedSearch : Carmona_2005_Eur.J.Pharmacol_517_186 |
| PubMedID: 15967428 |
| Title : Butyrylcholinesterase: an important new target in Alzheimer's disease therapy - Greig_2002_Int.Psychogeriatr_14_77 |
| Author(s) : Greig NH , Lahiri DK , Sambamurti K |
| Ref : Int Psychogeriatr , 14 :77 , 2002 |
| Abstract : Greig_2002_Int.Psychogeriatr_14_77 |
| ESTHER : Greig_2002_Int.Psychogeriatr_14_77 |
| PubMedSearch : Greig_2002_Int.Psychogeriatr_14_77 |
| PubMedID: 12636181 |
| Title : A new therapeutic target in Alzheimer's disease treatment: attention to butyrylcholinesterase - Greig_2001_Curr.Med.Res.Opin_17_159 |
| Author(s) : Greig NH , Utsuki T , Yu Q , Zhu X , Holloway HW , Perry T , Lee B , Ingram DK , Lahiri DK |
| Ref : Curr Med Res Opin , 17 :159 , 2001 |
| Abstract : Greig_2001_Curr.Med.Res.Opin_17_159 |
| ESTHER : Greig_2001_Curr.Med.Res.Opin_17_159 |
| PubMedSearch : Greig_2001_Curr.Med.Res.Opin_17_159 |
| PubMedID: 11900310 |
| Title : Cholinesterase inhibitors, beta-amyloid precursor protein and amyloid beta-peptides in Alzheimer's disease - Lahiri_2000_Acta.Neurol.Scand.Suppl_176_60 |
| Author(s) : Lahiri DK , Farlow MR , Hintz N , Utsuki T , Greig NH |
| Ref : Acta Neurologica Scandinavica Supplementum , 176 :60 , 2000 |
| Abstract : Lahiri_2000_Acta.Neurol.Scand.Suppl_176_60 |
| ESTHER : Lahiri_2000_Acta.Neurol.Scand.Suppl_176_60 |
| PubMedSearch : Lahiri_2000_Acta.Neurol.Scand.Suppl_176_60 |
| PubMedID: 11273593 |
| Title : Synthesis of novel phenserine-based-selective inhibitors of butyrylcholinesterase for Alzheimer's disease - Yu_1999_J.Med.Chem_42_1855 |
| Author(s) : Yu Q , Holloway HW , Utsuki T , Brossi A , Greig NH |
| Ref : Journal of Medicinal Chemistry , 42 :1855 , 1999 |
| Abstract : Yu_1999_J.Med.Chem_42_1855 |
| ESTHER : Yu_1999_J.Med.Chem_42_1855 |
| PubMedSearch : Yu_1999_J.Med.Chem_42_1855 |
| PubMedID: 10346939 |