CHMP-S-ethyl
can be synthetized as (Sp) or (P-) and (Rp) or (P+) stereoisomers
General
Type : Organophosphate,Sulfur Compound
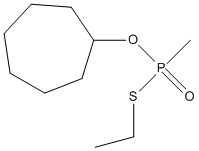
Chemical_Nomenclature : [ethylsulfanyl(methyl)phosphoryl]oxycycloheptane
Canonical SMILES : CCS[P](=O)(C)OC1CCCCCC1
InChI : InChI=1S\/C10H21O2PS\/c1-3-14-13(2,11)12-10-8-6-4-5-7-9-10\/h10H,3-9H2,1-2H3
InChIKey : PVRXMZHHAYEXFP-UHFFFAOYSA-N
Other name(s) : Cycloheptyl methyl S-ethylphosphonylthioate
MW : 263.31
Formula : C10H21O2PS
CAS_number :
PubChem : 85590433
UniChem : PVRXMZHHAYEXFP-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (3)
| Title : Aspartate 74 as a primary determinant in acetylcholinesterase governing specificity to cationic organophosphonates - Hosea_1996_Biochemistry_35_10995 |
| Author(s) : Hosea NA , Radic Z , Tsigelny I , Berman HA , Quinn DM , Taylor P |
| Ref : Biochemistry , 35 :10995 , 1996 |
| Abstract : Hosea_1996_Biochemistry_35_10995 |
| ESTHER : Hosea_1996_Biochemistry_35_10995 |
| PubMedSearch : Hosea_1996_Biochemistry_35_10995 |
| PubMedID: 8718893 |
| Title : Chiral reactions of acetylcholinesterase probed with enantiomeric methylphosphonothioates. Noncovalent determinants of enzyme chirality [published erratum appears in J Biol Chem 1989 Nov 25\;264(33):20154] - Berman_1989_J.Biol.Chem_264_3942 |
| Author(s) : Berman HA , Leonard K |
| Ref : Journal of Biological Chemistry , 264 :3942 , 1989 |
| Abstract : Berman_1989_J.Biol.Chem_264_3942 |
| ESTHER : Berman_1989_J.Biol.Chem_264_3942 |
| PubMedSearch : Berman_1989_J.Biol.Chem_264_3942 |
| PubMedID: 2917983 |
| Title : Chiral nature of covalent methylphosphonyl conjugates of acetylcholinesterase - Berman_1989_J.Biol.Chem_264_3951 |
| Author(s) : Berman HA , Decker MM |
| Ref : Journal of Biological Chemistry , 264 :3951 , 1989 |
| Abstract : Berman_1989_J.Biol.Chem_264_3951 |
| ESTHER : Berman_1989_J.Biol.Chem_264_3951 |
| PubMedSearch : Berman_1989_J.Biol.Chem_264_3951 |
| PubMedID: 2917984 |