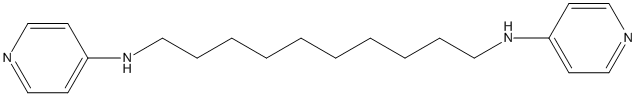
Bivalent-4-Aminopyridine-12i
12i dual-site binding strategy with two 4-Aminopyridine with tether length of 10 methylene units .(AChE IC50=152+/-30 nM) is at least 3000 times more potent than the corresponding monomer, and is even more potent than tacrine
General
Type : Pyridine,Alkyl linked bis-ligand
Chemical_Nomenclature : N,N'-dipyridin-4-yldecane-1,10-diamine
Canonical SMILES : C1=CN=CC=C1NCCCCCCCCCCNC2=CC=NC=C2
InChI : InChI=1S\/C20H30N4\/c1(3-5-7-13-23-19-9-15-21-16-10-19)2-4-6-8-14-24-20-11-17-22-18-12-20\/h9-12,15-18H,1-8,13-14H2,(H,21,23)(H,22,24)
InChIKey : NPFMKSWHBJHKPB-UHFFFAOYSA-N
Other name(s) : Bivalent 4-Aminopyridine 12i,BDBM10463,N,N-di-4-Pyridyl-1,10-diaminodecane bis(+)-tartaric acid salt,bis((2R,3R)-2,3-dihydroxybutanedioic acid),N-[10-(pyridin-4-ylamino)decyl]pyridin-4-amine
MW : 326.5
Formula : C20H30N4
CAS_number :
PubChem : 12069891
UniChem : NPFMKSWHBJHKPB-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : Bivalent-4-Aminopyridine-12i ligand of proteins in family: ACHE
Stucture :
Protein : human-ACHE
References (1)
| Title : Dual-site binding of bivalent 4-aminopyridine- and 4-aminoquinoline-based AChE inhibitors: contribution of the hydrophobic alkylene tether to monomer and dimer affinities - Han_1999_Bioorg.Med.Chem_7_2569 |
| Author(s) : Han YF , Li CP , Chow E , Wang H , Pang YP , Carlier PR |
| Ref : Bioorganic & Medicinal Chemistry , 7 :2569 , 1999 |
| Abstract : Han_1999_Bioorg.Med.Chem_7_2569 |
| ESTHER : Han_1999_Bioorg.Med.Chem_7_2569 |
| PubMedSearch : Han_1999_Bioorg.Med.Chem_7_2569 |
| PubMedID: 10632067 |