ASS234
multipotent cholinesterase/monoamine oxidase inhibitors for the treatment of Alzheimer's disease.
General
Type : Multitarget,Monoamine-oxidase-inhibitor,Alkyl linked bis-ligand,Piperidine,Indole
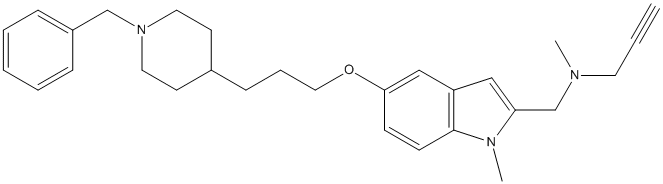
Chemical_Nomenclature : N-[[5-[3-(1-benzylpiperidin-4-yl)propoxy]-1-methylindol-2-yl]methyl]-N-methylprop-2-yn-1-amine
Canonical SMILES : CN1C2=C(C=C(C=C2)OCCCC3CCN(CC3)CC4=CC=CC=C4)C=C1CN(C)CC#C
InChI : InChI=1S\/C29H37N3O\/c1-4-16-30(2)23-27-20-26-21-28(12-13-29(26)31(27)3)33-19-8-11-24-14-17-32(18-15-24)22-25-9-6-5-7-10-25\/h1,5-7,9-10,12-13,20-21,24H,8,11,14-19,22-23H2,2-3H3
InChIKey : ADCBAOTWERXLAP-UHFFFAOYSA-N
Other name(s) : CHEMBL1929421,D0C1LI,GTPL7369,SCHEMBL6847307,ASS-234
MW : 443.63
Formula : C29H37N3O
CAS_number :
PubChem : 53378764
UniChem : ADCBAOTWERXLAP-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (11)
| Title : Highlights of ASS234: a novel and promising therapeutic agent for Alzheimer's disease therapy - Romero_2020_Neural.Regen.Res_15_30 |
| Author(s) : Romero A , Marco-Contelles J , Ramos E |
| Ref : Neural Regen Res , 15 :30 , 2020 |
| Abstract : Romero_2020_Neural.Regen.Res_15_30 |
| ESTHER : Romero_2020_Neural.Regen.Res_15_30 |
| PubMedSearch : Romero_2020_Neural.Regen.Res_15_30 |
| PubMedID: 31535639 |
| Title : Multitarget-Directed Ligands Combining Cholinesterase and Monoamine Oxidase Inhibition with Histamine H3 R Antagonism for Neurodegenerative Diseases - Bautista-Aguilera_2017_Angew.Chem.Int.Ed.Engl_56_12765 |
| Author(s) : Bautista-Aguilera OM , Hagenow S , Palomino-Antolin A , Farre-Alins V , Ismaili L , Joffrin PL , Jimeno ML , Soukup O , Janockova J , Kalinowsky L , Proschak E , Iriepa I , Moraleda I , Schwed JS , Romero Martinez A , Lopez-Munoz F , Chioua M , Egea J , Ramsay RR , Marco-Contelles J , Stark H |
| Ref : Angew Chem Int Ed Engl , 56 :12765 , 2017 |
| Abstract : Bautista-Aguilera_2017_Angew.Chem.Int.Ed.Engl_56_12765 |
| ESTHER : Bautista-Aguilera_2017_Angew.Chem.Int.Ed.Engl_56_12765 |
| PubMedSearch : Bautista-Aguilera_2017_Angew.Chem.Int.Ed.Engl_56_12765 |
| PubMedID: 28861918 |
| Title : The proof-of-concept of ASS234: Peripherally administered ASS234 enters the central nervous system and reduces pathology in a male mouse model of Alzheimer disease - Serrano_2017_J.Psychiatry.Neurosci_42_59 |
| Author(s) : Serrano MP , Herrero-Labrador R , Futch HS , Serrano J , Romero A , Fernandez AP , Samadi A , Unzeta M , Marco-Contelles J , Martinez-Murillo R |
| Ref : J Psychiatry Neurosci , 42 :59 , 2017 |
| Abstract : Serrano_2017_J.Psychiatry.Neurosci_42_59 |
| ESTHER : Serrano_2017_J.Psychiatry.Neurosci_42_59 |
| PubMedSearch : Serrano_2017_J.Psychiatry.Neurosci_42_59 |
| PubMedID: 27636528 |
| Title : In-vitro and in-vivo evaluation of the modulatory effects of the multitarget compound ASS234 on the monoaminergic system - Esteban_2017_J.Pharm.Pharmacol_69_314 |
| Author(s) : Esteban G , Van Schoors J , Sun P , Van Eeckhaut A , Marco-Contelles J , Smolders I , Unzeta M |
| Ref : J Pharm Pharmacol , 69 :314 , 2017 |
| Abstract : Esteban_2017_J.Pharm.Pharmacol_69_314 |
| ESTHER : Esteban_2017_J.Pharm.Pharmacol_69_314 |
| PubMedSearch : Esteban_2017_J.Pharm.Pharmacol_69_314 |
| PubMedID: 28134992 |
| Title : ASS234, As a New Multi-Target Directed Propargylamine for Alzheimer's Disease Therapy - Marco-Contelles_2016_Front.Neurosci_10_294 |
| Author(s) : Marco-Contelles J , Unzeta M , Bolea I , Esteban G , Ramsay RR , Romero A , Martinez-Murillo R , Carreiras MC , Ismaili L |
| Ref : Front Neurosci , 10 :294 , 2016 |
| Abstract : Marco-Contelles_2016_Front.Neurosci_10_294 |
| ESTHER : Marco-Contelles_2016_Front.Neurosci_10_294 |
| PubMedSearch : Marco-Contelles_2016_Front.Neurosci_10_294 |
| PubMedID: 27445665 |
| Title : Upregulation of Antioxidant Enzymes by ASS234, a Multitarget Directed Propargylamine for Alzheimer's Disease Therapy - |
| Author(s) : Ramos E , Romero A , Marco-Contelles J , Del Pino J |
| Ref : CNS Neurosci Ther , 22 :799 , 2016 |
| PubMedID: 27380946 |
| Title : Kinetic and structural analysis of the irreversible inhibition of human monoamine oxidases by ASS234, a multi-target compound designed for use in Alzheimer's disease - Esteban_2014_Biochim.Biophys.Acta_1844_1104 |
| Author(s) : Esteban G , Allan J , Samadi A , Mattevi A , Unzeta M , Marco-Contelles J , Binda C , Ramsay RR |
| Ref : Biochimica & Biophysica Acta , 1844 :1104 , 2014 |
| Abstract : Esteban_2014_Biochim.Biophys.Acta_1844_1104 |
| ESTHER : Esteban_2014_Biochim.Biophys.Acta_1844_1104 |
| PubMedSearch : Esteban_2014_Biochim.Biophys.Acta_1844_1104 |
| PubMedID: 24642166 |
| Title : Wnt signaling pathway, a potential target for Alzheimer's disease treatment, is activated by a novel multitarget compound ASS234 - |
| Author(s) : Del Pino J , Ramos E , Aguilera OM , Marco-Contelles J , Romero A |
| Ref : CNS Neurosci Ther , 20 :568 , 2014 |
| PubMedID: 24712554 |
| Title : Effects of novel monoamine oxidases and cholinesterases targeting compounds on brain neurotransmitters and behavior in rat model of vascular dementia - Stasiak_2014_Curr.Pharm.Des_20_161 |
| Author(s) : Stasiak A , Mussur M , Unzeta M , Samadi A , Marco-Contelles JL , Fogel WA , Marco-Contelles J |
| Ref : Curr Pharm Des , 20 :161 , 2014 |
| Abstract : Stasiak_2014_Curr.Pharm.Des_20_161 |
| ESTHER : Stasiak_2014_Curr.Pharm.Des_20_161 |
| PubMedSearch : Stasiak_2014_Curr.Pharm.Des_20_161 |
| PubMedID: 23701539 |
| Title : Multipotent, permeable drug ASS234 inhibits Abeta aggregation, possesses antioxidant properties and protects from Abeta-induced apoptosis in vitro - Bolea_2013_Curr.Alzheimer.Res_10_797 |
| Author(s) : Bolea I , Gella A , Monjas L , Perez C , Rodriguez-Franco MI , Marco-Contelles J , Samadi A , Unzeta M |
| Ref : Curr Alzheimer Res , 10 :797 , 2013 |
| Abstract : Bolea_2013_Curr.Alzheimer.Res_10_797 |
| ESTHER : Bolea_2013_Curr.Alzheimer.Res_10_797 |
| PubMedSearch : Bolea_2013_Curr.Alzheimer.Res_10_797 |
| PubMedID: 23919774 |
| Title : Synthesis, biological evaluation, and molecular modeling of donepezil and N-[(5-(benzyloxy)-1-methyl-1H-indol-2-yl)methyl]-N-methylprop-2-yn-1-amine hybrids as new multipotent cholinesterase\/monoamine oxidase inhibitors for the treatment of Alzheimer's disease - Bolea_2011_J.Med.Chem_54_8251 |
| Author(s) : Bolea I , Juarez-Jimenez J , de los Rios C , Chioua M , Pouplana R , Luque FJ , Unzeta M , Marco-Contelles J , Samadi A |
| Ref : Journal of Medicinal Chemistry , 54 :8251 , 2011 |
| Abstract : Bolea_2011_J.Med.Chem_54_8251 |
| ESTHER : Bolea_2011_J.Med.Chem_54_8251 |
| PubMedSearch : Bolea_2011_J.Med.Chem_54_8251 |
| PubMedID: 22023459 |