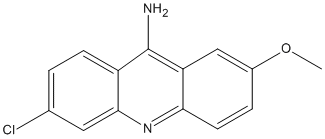
9-Amino-6-chloro-2-methoxyacridine
General
Type : Acridine,Derivative of Tacrine
Chemical_Nomenclature : 6-chloro-2-methoxyacridin-9-amine
Canonical SMILES : CC(=O)NC1=C(C2=CC3=CC=CC=C3C4=C2C5=C(C=CC=C51)C=C4)O
InChI : InChI=1S\/C22H15NO2\/c1-12(24)23-21-17-8-4-6-13-9-10-16-15-7-3-2-5-14(15)11-18(22(21)25)20(16)19(13)17\/h2-11,25H,1H3,(H,23,24)
InChIKey : CGVDLHRSVYTJKP-UHFFFAOYSA-N
Other name(s) : ACMA,G 185,6-Chloro-2-methoxy-9-acridinamine,NSC 15300
MW : 258.70
Formula : C14H11ClN2O
CAS_number : 3548-09-2
PubChem : 190803
UniChem : CGVDLHRSVYTJKP-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : 9-Amino-6-chloro-2-methoxyacridine ligand of proteins in family: Carboxylesterase || Carb_B_Chordata
Stucture :
Protein :
References (2)
| Title : Inhibition of human carboxylesterases hCE1 and hiCE by cholinesterase inhibitors - Tsurkan_2013_Chem.Biol.Interact_203_226 |
| Author(s) : Tsurkan LG , Hatfield MJ , Edwards CC , Hyatt JL , Potter PM |
| Ref : Chemico-Biological Interactions , 203 :226 , 2013 |
| Abstract : Tsurkan_2013_Chem.Biol.Interact_203_226 |
| ESTHER : Tsurkan_2013_Chem.Biol.Interact_203_226 |
| PubMedSearch : Tsurkan_2013_Chem.Biol.Interact_203_226 |
| PubMedID: 23123248 |
| Title : Mammalian carboxylesterases: from drug targets to protein therapeutics - Redinbo_2005_Drug.Discov.Today_10_313 |
| Author(s) : Redinbo MR , Potter PM |
| Ref : Drug Discov Today , 10 :313 , 2005 |
| Abstract : Redinbo_2005_Drug.Discov.Today_10_313 |
| ESTHER : Redinbo_2005_Drug.Discov.Today_10_313 |
| PubMedSearch : Redinbo_2005_Drug.Discov.Today_10_313 |
| PubMedID: 15749280 |