6FSD-10
combines a mosquito versus human AChE selectivity with a high potency also for the resistance-conferring mutation G122S (G119S)
General
Type : Piperidine
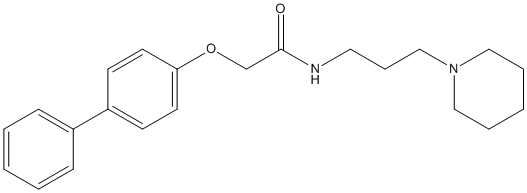
Chemical_Nomenclature : 2-(4-phenylphenoxy)-N-(3-piperidin-1-ylpropyl)acetamide
Canonical SMILES : C1CCN(CC1)CCCNC(=O)COC2=CC=C(C=C2)C3=CC=CC=C3
InChI : InChI=1S\/C22H28N2O2\/c25-22(23-14-7-17-24-15-5-2-6-16-24)18-26-21-12-10-20(11-13-21)19-8-3-1-4-9-19\/h1,3-4,8-13H,2,5-7,14-18H2,(H,23,25)
InChIKey : IYYRFRHVGUOBGU-UHFFFAOYSA-N
Other name(s) : 2-(4-Biphenylyloxy)-N-[3-(1-piperidinyl)propyl]-acetamide hydrochloride
MW : 352.47
Formula : C22H28N2O2
CAS_number :
PubChem : 9405885
UniChem : IYYRFRHVGUOBGU-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : 6FSD-10 ligand of proteins in family: ACHE
Stucture : 6FSD Mus musculus acetylcholinesterase in complex with 2-(4-Biphenylyloxy)-N-[3-(1-piperidinyl)propyl]-acetamide hydrochloride (10)
Protein : anoga-ACHE1
References (1)
| Title : Noncovalent Inhibitors of Mosquito Acetylcholinesterase 1 with Resistance-Breaking Potency - Knutsson_2018_J.Med.Chem_61_10545 |
| Author(s) : Knutsson S , Engdahl C , Kumari R , Forsgren N , Lindgren C , Kindahl T , Kitur S , Wachira L , Kamau L , Ekstrom F , Linusson A |
| Ref : Journal of Medicinal Chemistry , 61 :10545 , 2018 |
| Abstract : Knutsson_2018_J.Med.Chem_61_10545 |
| ESTHER : Knutsson_2018_J.Med.Chem_61_10545 |
| PubMedSearch : Knutsson_2018_J.Med.Chem_61_10545 |
| PubMedID: 30339371 |
| Gene_locus related to this paper: mouse-ACHE |