Clevidipine
General
Type : Not A\/B H target || Pro-Drug || Butyrate || Drug
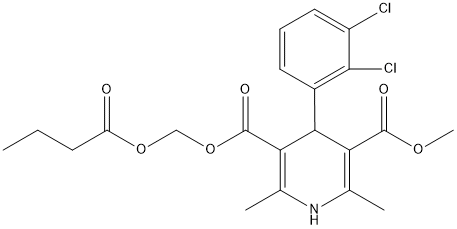
Chemical_Nomenclature : 5-O-(butanoyloxymethyl) 3-O-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
Canonical SMILES : CCCC(=O)OCOC(=O)C1=C(NC(=C(C1C2=C(C(=CC=C2)Cl)Cl)C(=O)OC)C)C
InChI : InChI=1S\/C21H23Cl2NO6\/c1-5-7-15(25)29-10-30-21(27)17-12(3)24-11(2)16(20(26)28-4)18(17)13-8-6-9-14(22)19(13)23\/h6,8-9,18,24H,5,7,10H2,1-4H3
InChIKey : KPBZROQVTHLCDU-UHFFFAOYSA-N
Other name(s) : Clevidipine butyrate, Cleviprex, Clevelox, SCHEMBL115522, CHEMBL1237132, CHEBI:135738
Target
Families : No family
References (1)
| Title : In vitro hydrolysis rate and protein binding of clevidipine, a new ultrashort-acting calcium antagonist metabolised by esterases, in different animal species and man - Ericsson_1999_Eur.J.Pharm.Sci_8_29 |
| Author(s) : Ericsson H , Tholander B , Regardh CG |
| Ref : Eur J Pharm Sci , 8 :29 , 1999 |
| Abstract : Ericsson_1999_Eur.J.Pharm.Sci_8_29 |
| ESTHER : Ericsson_1999_Eur.J.Pharm.Sci_8_29 |
| PubMedSearch : Ericsson_1999_Eur.J.Pharm.Sci_8_29 |
| PubMedID: 10072476 |