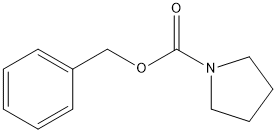
1-Cbz-pyrrolidine
General
Type : Epoxide || Benzyloxycarbonyl || Carbamate
Chemical_Nomenclature : benzyl pyrrolidine-1-carboxylate
Canonical SMILES : C1CCN(C1)C(=O)OCC2=CC=CC=C2
InChI : InChI=1S\/C12H15NO2\/c14-12(13-8-4-5-9-13)15-10-11-6-2-1-3-7-11\/h1-3,6-7H,4-5,8-10H2
InChIKey : VALPXRNQZYGXPS-UHFFFAOYSA-N
Other name(s) : N-benzyloxycarbonyl-3,4-epoxy-pyrrolidine, Benzyl Pyrrolidine-1-carboxylate, 1-Pyrrolidinecarboxylic acid, phenylmethyl ester, MFCD18206031, SCHEMBL1000788
Target
Families : Epoxide_hydrolase
References (2)
| Title : Enantioselective Hydrolysis of Racemic and Meso-Epoxides with Recombinant Escherichia coli Expressing Epoxide Hydrolase from Sphingomonas sp. HXN-200: Preparation of Epoxides and Vicinal Diols in High ee and High Concentration - Wu_2013_ACS.Catal_3_752 |
| Author(s) : Wu S , Li A , Chin YS , Li Z |
| Ref : ACS Catal , 3 :752 , 2013 |
| Abstract : Wu_2013_ACS.Catal_3_752 |
| ESTHER : Wu_2013_ACS.Catal_3_752 |
| PubMedSearch : Wu_2013_ACS.Catal_3_752 |
| PubMedID: |
| Gene_locus related to this paper: 9sphn-a0a191xv39 |
| Title : Highly enantioselective hydrolysis of alicyclic meso-epoxides with a bacterial epoxide hydrolase from Sphingomonas sp. HXN-200: simple syntheses of alicyclic vicinal trans-diols - Chang_2003_Chem.Commun.(Camb)__960 |
| Author(s) : Chang D , Wang Z , Heringa MF , Wirthner R , Witholt B , Li Z |
| Ref : Chem Commun (Camb) , :960 , 2003 |
| Abstract : Chang_2003_Chem.Commun.(Camb)__960 |
| ESTHER : Chang_2003_Chem.Commun.(Camb)__960 |
| PubMedSearch : Chang_2003_Chem.Commun.(Camb)__960 |
| PubMedID: 12744319 |
| Gene_locus related to this paper: 9sphn-a0a191xv39 |