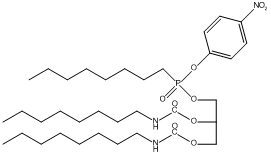
l,2-Dioctylcarbamoylglycero-3-O-p-nitrophenyloctylphosphonate
potent inhibitors of lipase Phosphonate analogues of triacylglycerols
General
Type : Organophosphate,Analogue of substrate,Lipase inhibitor,Triacylglycerol,pNP
Chemical_Nomenclature : [3-[(4-nitrophenoxy)-octyl-oxidophosphaniumyl]oxy-2-(octylcarbamoyloxy)propyl] N-octylcarbamate
Canonical SMILES : CCCCCCCCNC(=O)OCC(CO[P+](CCCCCCCC)([O-])OC1=CC=C(C=C1)[N+](=O)[O-])OC(=O)NCCCCCCCC
InChI : InChI=1S\/C35H62N3O9P\/c1-4-7-10-13-16-19-26-36-34(39)44-29-33(46-35(40)37-27-20-17-14-11-8-5-2)30-45-48(43,28-21-18-15-12-9-6-3)47-32-24-22-31(23-25-32)38(41)42\/h22-25,33H,4-21,26-30H2,1-3H3,(H,36,39)(H,37,40)
InChIKey : FBWOBOUTECHNKF-UHFFFAOYSA-N
Other name(s) : Octylphosphonic acid 4-nitrophenyl 2,3-bis(octylcarbamoyloxy)propyl ester
MW : 699.9
Formula : C35H62N3O9P
CAS_number :
PubChem : 101066212
UniChem : FBWOBOUTECHNKF-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : l,2-Dioctylcarbamoylglycero-3-O-p-nitrophenyloctylphosphonate ligand of proteins in family: Cutinase || Bacterial_lip_FamI.6
Stucture :
Protein : fusso-cutas || stahy-lipas
References (2)
| Title : Chiral preference of cutinase in the reaction with phosphonate inhibitors - |
| Author(s) : Mannesse ML , De Haas GH , van der Hijden HT , Egmond MR , Verheij HM |
| Ref : Biochemical Society Transactions , 25 :165 , 1997 |
| PubMedID: 9056864 |
| Title : Phosphonate analogues of triacylglycerols are potent inhibitors of lipase - Mannesse_1995_Biochim.Biophys.Acta_1259_56 |
| Author(s) : Mannesse ML , Boots JW , Dijkman R , Slotboom AJ , van der Hijden HT , Egmond MR , Verheij HM , De Haas GH |
| Ref : Biochimica & Biophysica Acta , 1259 :56 , 1995 |
| Abstract : Mannesse_1995_Biochim.Biophys.Acta_1259_56 |
| ESTHER : Mannesse_1995_Biochim.Biophys.Acta_1259_56 |
| PubMedSearch : Mannesse_1995_Biochim.Biophys.Acta_1259_56 |
| PubMedID: 7492616 |
| Gene_locus related to this paper: fusso-cutas , stahy-lipas |