Z-Gly-Pro
Product of hydrolysis of Z-Gly-Pro-AMC (7-amido-4-methylcoumarin) a fluorogenic substrate
General
Type : Peptide,Pyrrolidine,Benzyloxycarbonyl
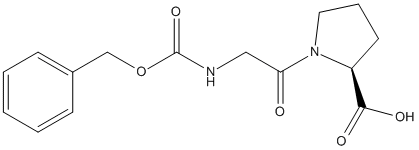
Chemical_Nomenclature : (2S)-1-[2-(phenylmethoxycarbonylamino)acetyl]pyrrolidine-2-carboxylic acid
Canonical SMILES : C1CC(N(C1)C(=O)CNC(=O)OCC2=CC=CC=C2)C(=O)O
InChI : InChI=1S\/C15H18N2O5\/c18-13(17-8-4-7-12(17)14(19)20)9-16-15(21)22-10-11-5-2-1-3-6-11\/h1-3,5-6,12H,4,7-10H2,(H,16,21)(H,19,20)\/t12-\/m0\/s1
InChIKey : ZTUKZKYDJMGJDC-LBPRGKRZSA-N
Other name(s) : Z-Gly-Pro-OH,N-Cbz-glycyl-L-proline,Z-Glycyl-L-proline,(S)-1-(2-(((Benzyloxy)carbonyl)amino)acetyl)pyrrolidine-2-carboxylic acid
MW : 306.31
Formula : C15H18N2O5
CAS_number : 1160-54-9
PubChem : 439540
UniChem : ZTUKZKYDJMGJDC-LBPRGKRZSA-N
IUPHAR :
Wikipedia :
Target
Families : Z-Gly-Pro ligand of proteins in family: S9N_PPCE_Peptidase_S9
Stucture : 1E8M Prolyl oligopeptidase from pig brain mutant covalently bound Z pro prolinal
Protein : pig-ppce
References (1)
| Title : Structures of prolyl oligopeptidase substrate\/inhibitor complexes. Use of inhibitor binding for titration of the catalytic histidine residue - Fulop_2001_J.Biol.Chem_276_1262 |
| Author(s) : Fulop V , Szeltner Z , Renner V , Polgar L |
| Ref : Journal of Biological Chemistry , 276 :1262 , 2001 |
| Abstract : Fulop_2001_J.Biol.Chem_276_1262 |
| ESTHER : Fulop_2001_J.Biol.Chem_276_1262 |
| PubMedSearch : Fulop_2001_J.Biol.Chem_276_1262 |
| PubMedID: 11031266 |
| Gene_locus related to this paper: pig-ppce |