Tolclofos-methyl
General
Type : Organophosphate,Fungicide,Sulfur Compound
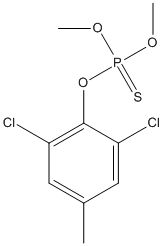
Chemical_Nomenclature : (2,6-dichloro-4-methylphenoxy)-dimethoxy-sulfanylidene-$l^{5}-phosphane
Canonical SMILES : CC1=CC(=C(C(=C1)Cl)OP(=S)(OC)OC)Cl
InChI : InChI=1S\/C9H11Cl2O3PS\/c1-6-4-7(10)9(8(11)5-6)14-15(16,12-2)13-3\/h4-5H,1-3H3
InChIKey : OBZIQQJJIKNWNO-UHFFFAOYSA-N
Other name(s) : Tolclofos-methyl,Tolclophos-methyl,Risolex,Rizolex,Basilex,Toclofos-methyl
MW : 301.12
Formula : C9H11Cl2O3PS
CAS_number : 57018-04-9
PubChem : 91664
UniChem : OBZIQQJJIKNWNO-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
References (3)
| Title : Oxidative Activation and Degradation of Organophosphorus Pesticides Mediated by Iron Porphyrins - Fujisawa_2005_J.Pestic.Sci_30_103 |
| Author(s) : Fujisawa T , Katagi T |
| Ref : Journal of Pesticide Science , 30 :103 , 2005 |
| Abstract : Fujisawa_2005_J.Pestic.Sci_30_103 |
| ESTHER : Fujisawa_2005_J.Pestic.Sci_30_103 |
| PubMedSearch : Fujisawa_2005_J.Pestic.Sci_30_103 |
| PubMedID: |
| Title : Evaluation of respiratory and cutaneous doses and urinary excretion of alkylphosphates by workers in greenhouses treated with omethoate, fenitrothion, and tolclofos-methyl - Aprea_2001_AIHAJ_62_87 |
| Author(s) : Aprea C , Sciarra G , Lunghini L , Centi L , Ceccarelli F |
| Ref : Aihaj , 62 :87 , 2001 |
| Abstract : Aprea_2001_AIHAJ_62_87 |
| ESTHER : Aprea_2001_AIHAJ_62_87 |
| PubMedSearch : Aprea_2001_AIHAJ_62_87 |
| PubMedID: 11258873 |
| Title : Spontaneous reactivation of mouse plasma cholinesterase after inhibition by various organophosphorus compounds - Matsubara_1984_J.Pharmacobiodyn_7_322 |
| Author(s) : Matsubara T , Horikoshi I |
| Ref : J Pharmacobiodyn , 7 :322 , 1984 |
| Abstract : Matsubara_1984_J.Pharmacobiodyn_7_322 |
| ESTHER : Matsubara_1984_J.Pharmacobiodyn_7_322 |
| PubMedSearch : Matsubara_1984_J.Pharmacobiodyn_7_322 |
| PubMedID: 6470929 |