Tetracyclic-carbamate-UW-MD-95
IC50 hAChE 3.6 microM; IC50 hBChE 5.2 nM
General
Type : Carbamate,Natural_modified,Derivative of Evodiamine,Isoquinoline,Quinazoline
Chemical_Nomenclature : (13-methyl-5,6,8,13a-tetrahydroisoquinolino[1,2-b]quinazolin-10-yl) N-heptylcarbamate
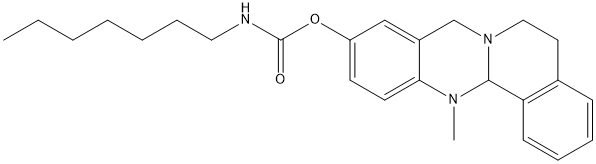
Canonical SMILES : CCCCCCCNC(=O)OC1=CC2=C(C=C1)N(C3C4=CC=CC=C4CCN3C2)C
InChI : InChI=1S\/C25H33N3O2\/c1-3-4-5-6-9-15-26-25(29)30-21-12-13-23-20(17-21)18-28-16-14-19-10-7-8-11-22(19)24(28)27(23)2\/h7-8,10-13,17,24H,3-6,9,14-16,18H2,1-2H3,(H,26,29)
InChIKey : NRSCUZWEMZYPRV-UHFFFAOYSA-N
Other name(s) : CHEMBL2152545,BDBM50392999,PD168451,UW-MD-95
MW : 407.5
Formula : C25H33N3O2
CAS_number :
PubChem : 60165327
UniChem : NRSCUZWEMZYPRV-UHFFFAOYSA-N
IUPHAR :
Wikipedia :
Target
Families : Tetracyclic-carbamate-UW-MD-95 ligand of proteins in family: BCHE
Stucture :
Protein : human-BCHE
References (3)
| Title : Selective Pseudo-irreversible Butyrylcholinesterase Inhibitors Transferring Antioxidant Moieties to the Enzyme Show Pronounced Neuroprotective Efficacy In Vitro and In Vivo in an Alzheimer's Disease Mouse Model - Scheiner_2021_J.Med.Chem_64_9302 |
| Author(s) : Scheiner M , Hoffmann M , He F , Poeta E , Chatonnet A , Monti B , Maurice T , Decker M |
| Ref : Journal of Medicinal Chemistry , 64(13): :9302 , 2021 |
| Abstract : Scheiner_2021_J.Med.Chem_64_9302 |
| ESTHER : Scheiner_2021_J.Med.Chem_64_9302 |
| PubMedSearch : Scheiner_2021_J.Med.Chem_64_9302 |
| PubMedID: 34152756 |
| Title : Highly Selective Butyrylcholinesterase Inhibitors with Tunable Duration of Action by Chemical Modification of Transferable Carbamate Units Exhibit Pronounced Neuroprotective Effect in an Alzheimer's Disease Mouse Model - Hoffmann_2019_J.Med.Chem_62_9116 |
| Author(s) : Hoffmann M , Stiller C , Endres E , Scheiner M , Gunesch S , Sotriffer C , Maurice T , Decker M |
| Ref : Journal of Medicinal Chemistry , 62 :9116 , 2019 |
| Abstract : Hoffmann_2019_J.Med.Chem_62_9116 |
| ESTHER : Hoffmann_2019_J.Med.Chem_62_9116 |
| PubMedSearch : Hoffmann_2019_J.Med.Chem_62_9116 |
| PubMedID: 31609115 |
| Title : Neuroprotective Tri- and Tetracyclic BChE Inhibitors Releasing Reversible Inhibitors upon Carbamate Transfer - Darras_2012_ACS.Med.Chem.Lett_3_914 |
| Author(s) : Darras FH , Kling B , Heilmann J , Decker M |
| Ref : ACS Med Chem Lett , 3 :914 , 2012 |
| Abstract : Darras_2012_ACS.Med.Chem.Lett_3_914 |
| ESTHER : Darras_2012_ACS.Med.Chem.Lett_3_914 |
| PubMedSearch : Darras_2012_ACS.Med.Chem.Lett_3_914 |
| PubMedID: 24900407 |